A research group led by Prof. CHEN Liang at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences (CAS) has designed a high-entropy electrocatalyst, achieving high-efficiency production of hydrogen and valuable glycerol chemicals.
The study was published in Nature Nanotechnology.
Hydrogen is a versatile energy carrier and industrial gas with wide-ranging applications. Electrolyzing water to produce hydrogen has emerged as a cost-efficient and sustainable energy conversion and storage solution.
However, the low activity and high overpotential of oxygen evolution reactions (OERs) at the anode lead to inefficient energy conversion and elevated operational costs, thus limiting the commercial viability of water electrolysis for hydrogen production.
Researchers at NIMTE designed and synthesized a high-entropy nanostructured PtCuCoNiMn catalyst.
Glycerate, a valuable chemical product, can thus be generated from glycerol via a cascade electro-oxidation reaction. Compared with traditional OERs, this electro-oxidation reaction is more energy efficient.
Relevant analysis revealed that introducing Cu, Pt, Co, Mn, and Ni enhances both activity and glycerate selectivity. Under high current densities of 200 mA cm−2, the developed catalyst for glycerate production showed an exceptional selectivity of 75.2%, indicating an excellent activity of electro-oxidation reactions.
When applied in an electrolyser, the catalyst exhibited outstanding stability, achieving high-performance glycerol electro-oxidation reaction for over 210 hours.
This work offers a sustainable and efficient approach for producing green hydrogen while synthesizing high-value chemicals through electrocatalysis, a dual-benefit solution that marks a significant step in achieving carbon peaking and carbon neutrality goals.
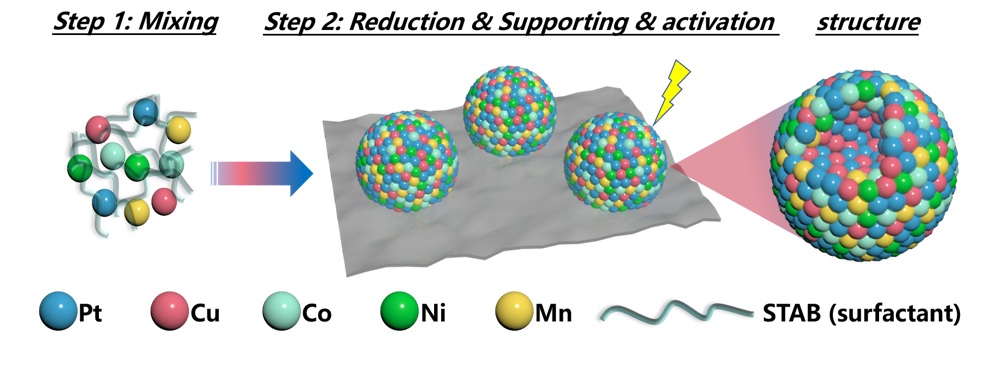
Synthesis of high-entropy nanostructured PtCuCoNiMn electrocatalysts (Image by NIMTE)
Contact
LIN Yichao
Ningbo Institute of Materials Technology and Engineering
E-mail: yclin@nimte.ac.cn

