Prof. LU Zhiyi, Prof. WANG Aiying and coworkers at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of Chinese Academy of Sciences (CAS), have revealed the corrosion mechanism of Br- on Ni-based anodes, contributing to the stability of seawater electrolysis.
The study was published in Nature Communications.
Electrolyzing seawater to produce hydrogen has been considered as a cost-efficient and sustainable energy conversion and storage strategy, which contributes to the goal of "peak carbon dioxide emission and carbon neutrality". However, the poor durability of anodes has limited the application of seawater electrolysis for hydrogen generation.
Based on previous studies on the stability of seawater electrolysis, researchers at NIMTE delved into the corrosion mechanism of anodes in seawater electrolysis. Besides Cl-, Br- was elucidated even more harmful to Ni-based anodes during seawater electrolysis.
Assessment results of electrochemical tests demonstrated the inferior corrosion resistance and faster corrosion kinetics of Ni-based anodes in Br--containing electrolytes, compared with those in Cl--containing electrolytes.
In-situ electrochemical measurements revealed that Cl- prefers to form localized corrosion with narrow-deep pits, while Br- tends to generate extensive corrosion with wide-shallow pits on Ni substrates.
By virtue of the density functional theory (DFT) calculations and nudged elastic band (NEB) simulations, this difference in corrosion behaviors can be attributed to the slower diffusion and the lower reaction energy of Br- compared with Cl- in the passivation layer.
Furthermore, in terms of the Ni-based electrodes with catalysts, such as NiFe-LDH, Br- can induce large-area exfoliation of the catalyst layer during electrolysis, leading to the rapid performance degradation.
Despite the trace amount (0.53 mM) in seawater, Br- exerts great influence on the corrosion behaviors of Ni substrates, thus deserving more attention.
The corrosion mechanism exploration of Br- and Cl- on Ni-based anodes in this study may shed light on the design and synthesis of anodes for durable and stable seawater electrolysis.
This work was supported by the National Key Research and Development Project (2021YFA1502200/Z.Y.L.), Ningbo Yongjiang Talent Introduction Programme (No. 2021A-036-B/Z.Y.L.), Bellwethers Project of Zhejiang Research and Development Plan (No. 2022C01158/Z.Y.L.), the Ningbo S&T Innovation 2025 Major Special Program (Nos. 2022Z205/Q.H.Y., 2020Z107/W.W.X.), National Natural Science Foundation of China (NOS. 22105214/W.W.X., 52201285/X.C.), and Natural Science Foundation of Ningbo (No., 20221JCGY010295/W.W.X.), etc.
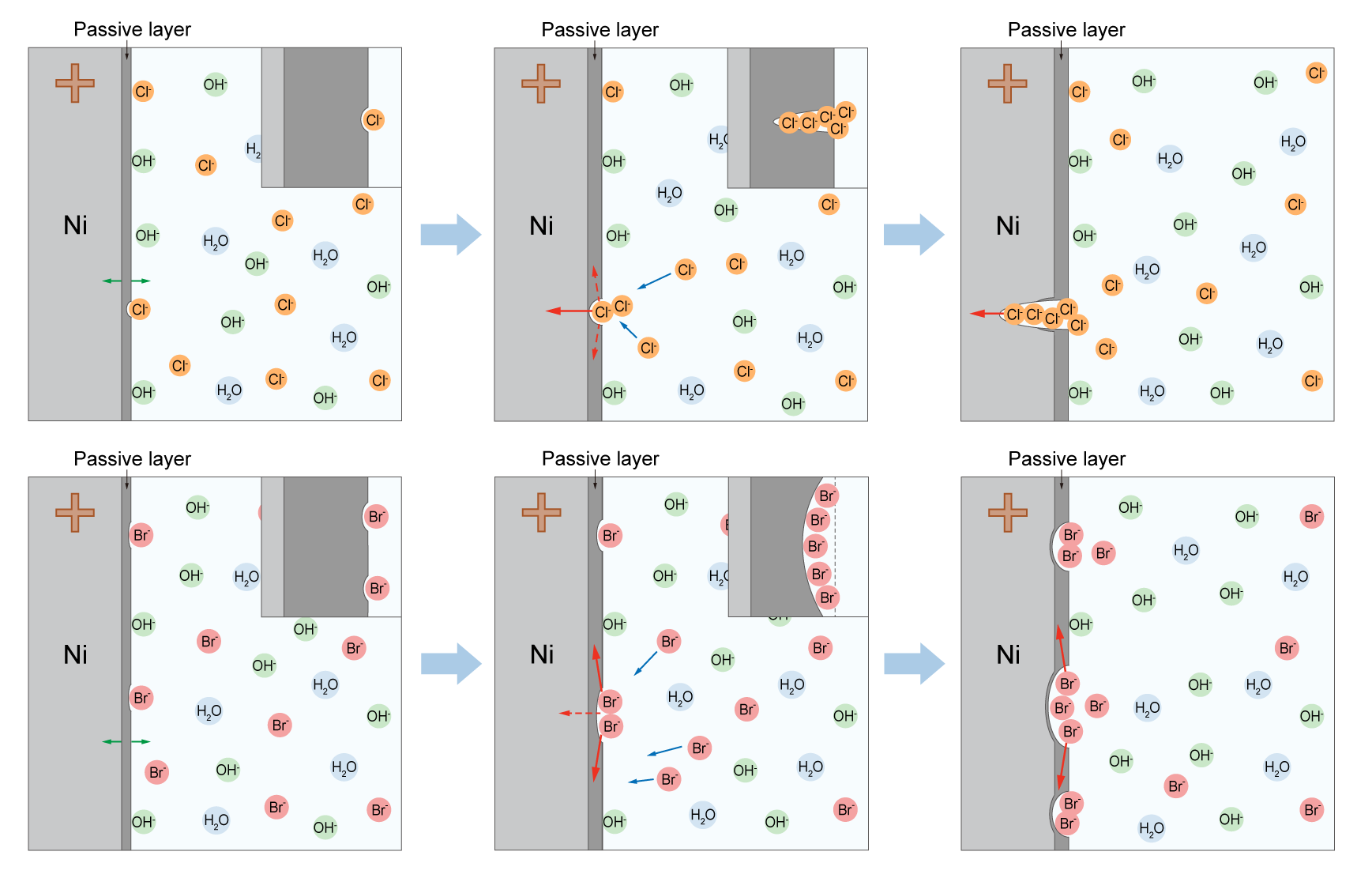
Fig. The corrosion processes of Ni substrates in Cl-/Br--containing electrolytes (Image by NIMTE)
Contact
LU Zhiyi
Ningbo Institute of Materials Technology and Engineering
Email: luzhiyi@nimte.ac.cn

