A research group led by Prof. WANG Liping at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of Chinese Academy of Sciences (CAS) has unveiled the enhanced oxygen reduction reaction (ORR) activity and biperiodic chemical trends of lanthanide-doped molybdenum disulfide (Ln-MoS2). The study was published in Nature Communications.
MoS2 holds a broad application prospect in catalysis, solid lubrication, optoelectronics, and other fields. Diverse lanthanide (Ln), e.g., Sm, Eu, Dy, Ho, Er, and Yb, etc., can be doped into MoS2 to modify physicochemical properties.
Reducing O2 into H2O, the oxygen reduction on the surfaces plays a crucial role in the performance and service life of Ln-MoS2-based functional materials, coatings and devices, such as the fuel cell efficiency and device galvanic corrosion. Exploring the ORR activity on the surface of Ln-MoS2 and its orbital-chemistry mechanism can provide guidance for the practical application design, precise performance regulation and effective protection of Ln-MoS2 systems.
By virtue of density-functional theory (DFT) calculations, researchers at NIMTE delved into the ORR process on all the 15 Ln-MoS2 (Ln = La ~ Lu) surfaces.
The doping of Ln considerably enhanced the ORR activity on Ln-MoS2 surfaces. In addition, an intriguing modulating biperiodic chemical trend of the ORR activity was observed.
Besides, the water effect on the loose MoS2/liquid interface was accurately simulated based on the thermodynamic statistics. Current-potential polarization curve simulations were also conducted to quantitatively reveal the ORR activity and effectively guide the related experiments.
In-depth electronic-structure analysis revealed that the enhancement of ORR activity can be attributed to a defect-state pairing mechanism, which selectively stabilizes the hydroxyl (OH) and hydroperoxyl (OOH) adsorbates on Ln-MoS2, thus significantly reducing the ORR energy barrier.
Moreover, a generic orbital-chemistry mechanism of Ln-MoS2 systems was proposed, contributing to explaining the intrinsic biperiodic trends observed in various electronic, thermodynamic, and kinetic properties.
This work may shed light on the design of superior Ln-MoS2-based materials and related systems, which shows a promising application and commercial prospect in electrocatalysts, optoelectronic nanodevices, and anti-corrosive coatings.
The research was supported by the National Natural Science Foundation of China (Grant Nos. U21A20127 and 22272192, L.W. and L.-F.H.), the Fund of Science and Technology on Surface Physics and Chemistry Laboratory (Grant No. XKFZ202101, L.-F.H.), the National Key Research and Development Project (Grant No. 2022YFB3402803, L.-F.H.), and the Natural Science Foundation of Ningbo (Grant No. 2021J229, L.-F.H.).
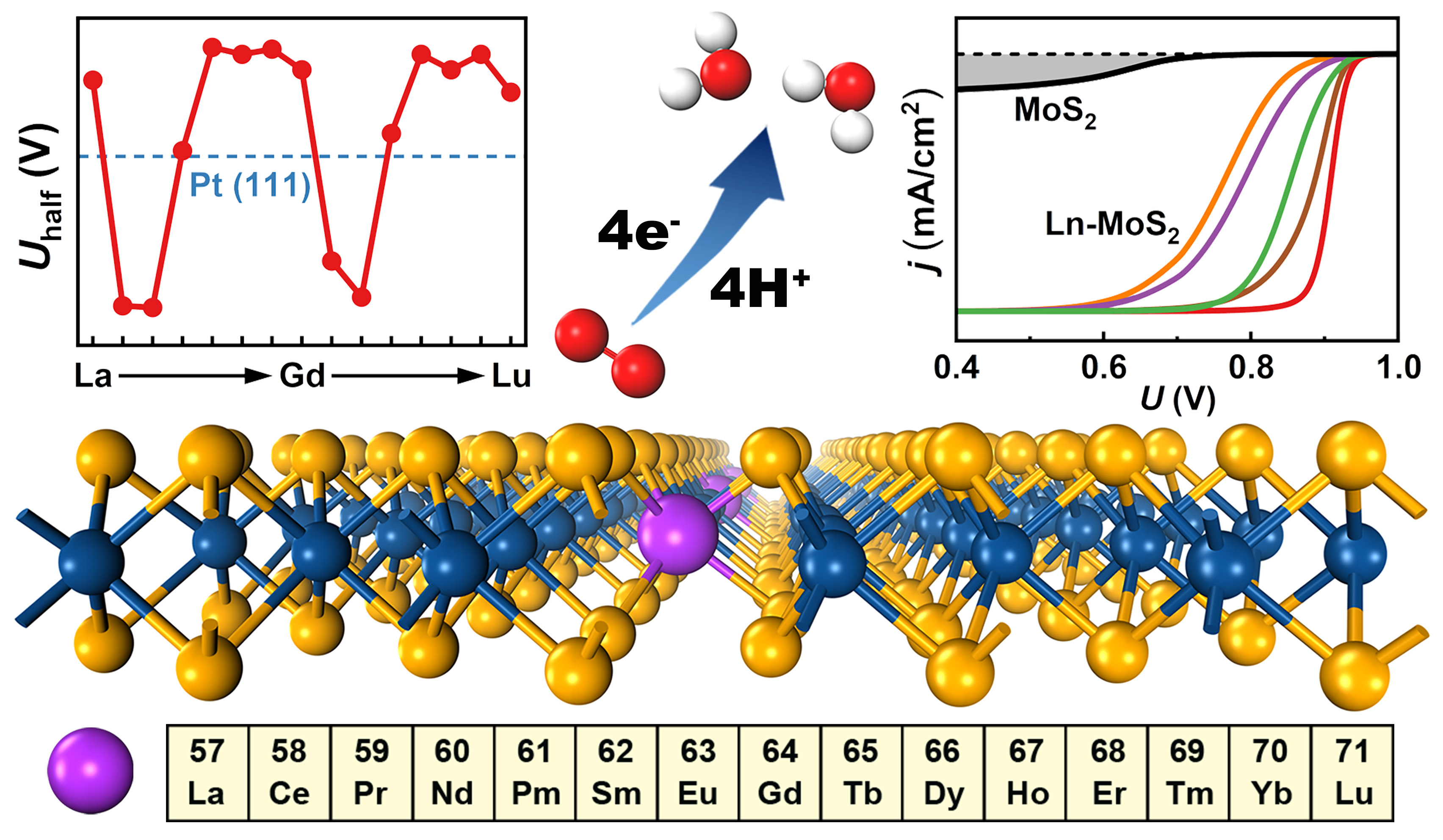
Fig. The biperiodic chemical trend and enhanced oxygen reduction reaction activity of Ln-MoS2 (Image by NIMTE)
Contact
HUANG Liangfeng
Ningbo Institute of Materials Technology and Engineering
E-mail: huangliangfeng@nimte.ac.cn

