Prof. CHEN Liang’s team at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of Chinese Academy of Sciences (CAS) observed that the addition of sulfate in electrolyte can greatly alleviate corrosion, thus enhancing the operating stability of seawater electrolysis for hydrogen production. The study was published in Angewandte Chemie International Edition.
Hydrogen can be generated through seawater electrolysis, which helps preserve freshwater supplies and thus has been considered as a sustainable energy conversion and storage strategy under extreme conditions. However, the highly concentrated chloride anions (Cl-) in seawater triggered severe anode corrosion.
To address this issue, scientists at NIMTE took nickel foam (NF) as a representative anode, and discovered that the addition of the cost-effective sulfate (SO42-) to the alkaline seawater electrolytic system can effectively retard the corrosion of chloride ions to the anode, thus significantly enhance the operation stability of NF anode.
Both theoretical simulations and in situ experiments demonstrated that sulfate anions can be preferentially adsorbed on anode surface to form a negative charge layer, which repulses the chloride ions away from the anode by virtue of electrostatic repulsion.
By virtue of the repulsive effect of additive SO42-, the nickel iron layered double hydroxide (NiFe-LDH) nanoarrays/NF anode showed excellent stability in a highly concentrated electrolyte with SO42-, which can achieve stable electrolysis for 500–1000 hours, 3–5 times than that worked in electrolyte without SO42-.
The repulsive effect of electrolyte additives demonstrated in this work may shed light on the further research on other active electrodes, and promote the scale-up of seawater electrolysis for hydrogen production.
This study was supported by the Ningbo S&T Innovation 2025 Major Special Program (Nos. 2020Z059 and 2020Z107), the BoXin project (No. BX20190339), the Natural Science Foundation of. Ningbo (Nos. 202003N4351), the China Postdoctoral Science Foundation (No. 2019M662124), the National Natural Science Foundation of China (NOs. 22002088, 22105214), the Shanghai Sailing Program (No.20YF1420500), and the Oceanic Interdisciplinary Program of Shanghai Jiao Tong University (No. SL2020MS007).
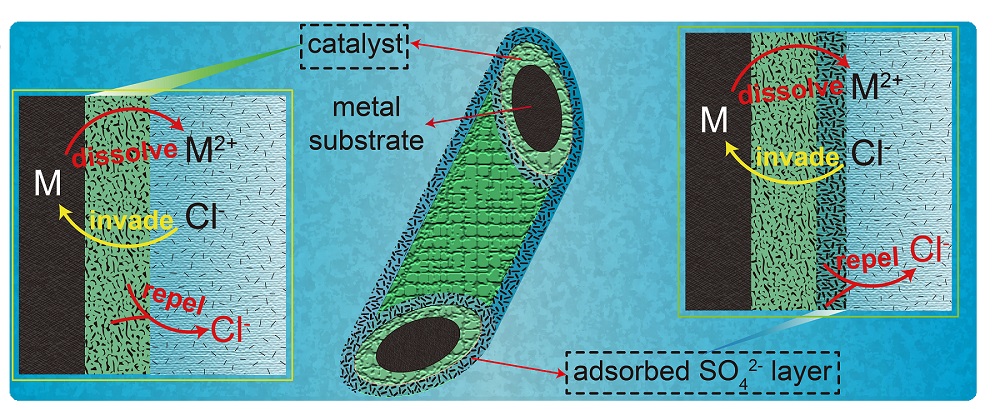
Fig. Protection of the metal substrate from Cl- corrosion (Image by NIMTE)
Contact
LU Zhiyi
Ningbo Institute of Materials Technology and Engineering
E-mail: luzhiyi@nimte.ac.cn

