A research group led by Prof. ZHANG Jian at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences (CAS), has demonstrated that 2,5-bis(hydroxymethyl)furan (BHMF) with high stability in strong alkali solvents is a novel feedstock superior to 5-hydroxymethylfurfural (HMF) to produce bio-based 2,5-furandicarboxylic acid (FDCA) by electrocatalysis with simultaneous generation of high-purity H2. The study was published in Applied Catalysis B: Environmental and applied for invention patents (CN202010242554.8, PCT/CN2020/082396).
The growing depletion of petrochemical resources and increasing environmental concerns urge scientists to pursue research on biomass conversion and utilization. Identified in 2004, FDCA is one of the Top-12 bio-based building blocks, which has extensive applications for polymer and pharmacy. Since 2016, the integrated HMF oxidation with water splitting to generate FDCA and H2 by electrocatalysis has drawn tremendous attention, due to the significantly improved energy utilization efficiency of a nearly 200 % Faradaic efficiency (FE). However, HMF is not thermally and chemically stable, limiting the product purity, storage and industrialization.
To address this issue, based on the previous cooperation with Zhejiang Sugar Energy Technology Co., Ltd. in mass production of BHMF by fixed-bed hydrogenation of HMF, Prof. ZHANG Jian and his colleagues proposed the aldehyde-free BHMF for electrocatalytic FDCA production for the first time.
Compared to HMF, BHMF is more attractive to achieve downstream derivatives with higher purity due to its excellent chemical stability and thermal properties (melting point: 74~77 oC for BHMF, 30~34 oC for HMF; boiling point: 275 oC for BHMF, 114~116 oC for HMF).
By virtue of facile electrodeposition and oxidative activation, a 3D standing CoOOH nanosheets decorated nickel foam was developed as a bifunctional electrode for BHMF electrooxidation and hydrogen evolution, which showed high efficiency and structural stability. The porous structure facilitated the charge transfer and mass diffusion, achieving complete BHMF conversion, pure H2 evolution as well as 90.2 % yield and 92.3 % FE toward FDCA generation. Based on the LSV current density and initial reaction rate, the calculated apparent activation energy (Ea) demonstrated an ultralow reaction energy barrier for BHMF conversion over CoOOH/Ni electrode, showing the superiority of electrocatalytic oxidation.
As a laboratory demonstration for practical production, a continuous flow cell with two 7X7 cm2 electrodes was employed to electrolyze BHMF into FDCA for the first time, achieving 0.92 g FDCA powder with high purity of nearly 100 % after simple acidification.
This study has also shed light on the cost reduction of storing and transporting biomass feedstock and the purity improvement of the end product, thus promoting the further development and application of biomass.
This work was supported by the National Natural Science Foundation of China (No. 22072170), Zhejiang Provincial Natural Science Foundation of China (No. LY19B030003, LQ19B060002), Ningbo Science and Technology Bureau (No. 2018B10056, 2019B10096), and Chinese Academy of Sciences (No. QYZDB-SSW-JSC037).
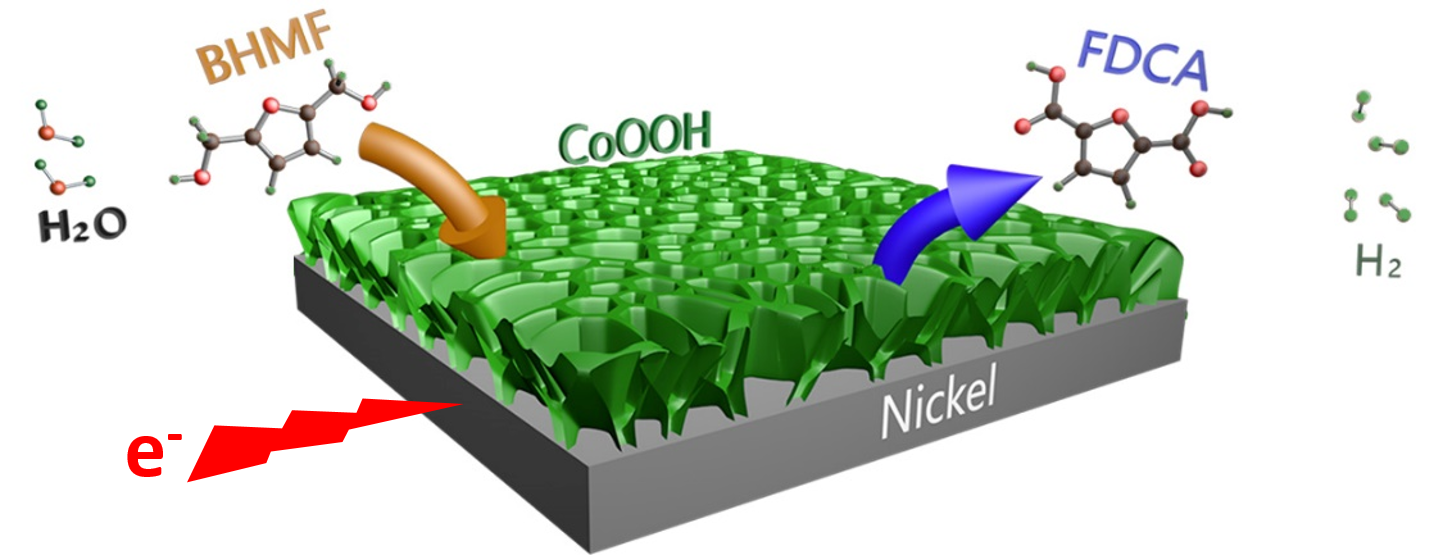
Fig. The simultaneous production of FDCA and H2 by virtue of BHMF (Image by NIMTE)
Contact
CHEN Chunlin
Ningbo Institute of Materials Technology and Engineering
E-mail: chenchunlin@nimte.ac.cn

