Prof. WU Aiguo’s team at the Cixi Institute of Biomedical Engineering, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences (CAS), developed a novel therapeutic method termed mechano-chemotherapy, which can efficiently overcome tumor drug-resistance. The study was published in Nano Today.
As one of the most common cancer treatment patterns, chemotherapy has been highlighted with superiority for cancer remission or even cure. However, the ability of tumor cells to develop drug resistance over time remains one of the major stumbling blocks in such a therapeutic strategy. In addition, drug release techniques (e.g. pH- and redox-induced carrier self-opening) are still in their infancy, owing to the existing barriers to achieve external stimulus responsive control.
To address this problem, researchers at NIMTE designed a controllable mechano-chemotherapeutic nanomaterial, integrating Zn0.2Fe2.8O4 magnetic nanoparticles (mNPs) and the Doxorubicin (DOX) anti-cancer drug into a poly(lactic-co-glycolic acid) (PLGA) carrier (DOX-Zn0.2Fe2.8O4-PLGA). Thanks to its superb magnetic response, the prepared nanomaterial can be easily controlled by an external rotating magnetic field (RMF). In the meantime, the ultra-high biocompatibility of the PLGA endows the nanomaterial with high stability in physiological environment.
Regarding to therapeutic outcomes, DOX-Zn0.2Fe2.8O4–PLGA with multi functionalities enabled safe and reliable controlled drug release, efficiently overcoming drug resistance. During the healing process, the tunable RMF equipment (45mT and 2000rpm in this work) as a magnetic switch was qualified to liberate the entrapped drug. Furthermore, the inner Zn0.2Fe2.8O4 mNPs generated a mechanical force under the external RMF, which therefore inflicted significant damage to tumour cells membrane alongside lysosome membrane and realized synergetic therapy.
The precise, non-invasive and remote mechano-chemotherapy has provided a novel therapeutic method to overcome the drug resistance of tumor cells and facilitate cancer cure, and shed light on the research of mechanical stimulation of other biological activities.
This work was supported by the National Natural Science Foundation of China (No. 51803228, 31971292), Zhejiang Provincial Natural Science Foundation of China (No. LGF18H180017) and Ningbo Natural Science Foundation of China (No. 2019A610192).
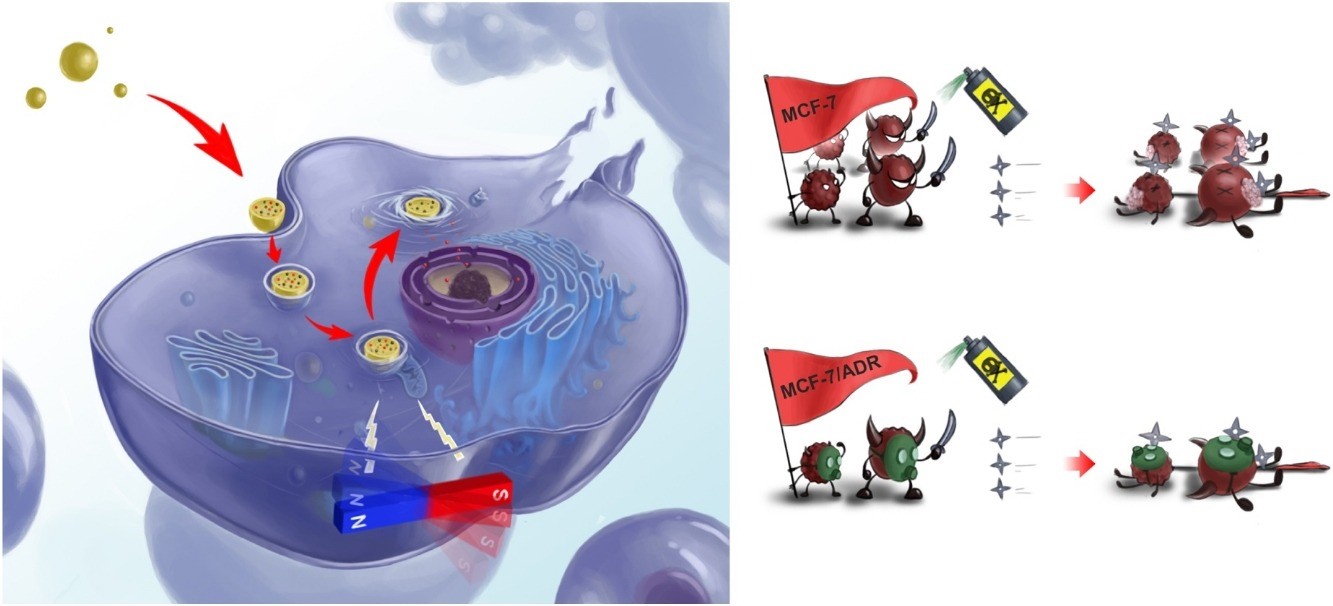
Fig. The illustration of the mechano-chemotherapy for killing tumor cells (Image by NIMTE)
Contact
YANG Fang
Ningbo Institute of Materials Technology and Engineering
E-mail: yangf@nimte.ac.cn

