A researcher group led by Prof. YANG Minghui at the Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences (CAS), discovered that zirconium nitride (ZrN) catalysts could be a superior alternative to expensive platinum (Pt) catalysts for oxygen reduction. The study was published in Nature Materials.
Most electrochemical energy devices, like fuel cells and metal-air batteries, need catalysts to boost power at acceptable rates. Oxygen reduction reaction (ORR) is one of the most fundamental reactions involved in electrochemical energy devices, which has greatly influenced the power delivery from devices.
Pt is currently the most widely used and commercial ORR catalyst. However, it is a scarcely available metal (37 ppb in Earth’s crust) with high cost (US$28.3 g-1 as the 2018 average price), which limits the large-scale applications of electrochemical energy devices.
To address this issue, the reaesrch group used a urea–glass route at moderate temperatures to prepare ZrN nanoparticles (NPs), which could substitute and even surpass the high-cost Pt as a catalyst for oxygen reduction under alkaline conditions.
As-synthesized ZrN NPs showed a high oxygen reduction performance with the same activity as that of the traditional Pt-on-carbon (Pt/C) commercial catalyst. Furthermore, in 0.1 M KOH solutions, both materials showed the same halfwave potential (E1/2 = 0.80 V) and ZrN exhibited a higher stability (ΔE1/2 = -3 mV) than the Pt/C catalyst (ΔE1/2 = -39 mV) after 1,000 ORR cycles. Besides, ZrN delivered a greater power density and cyclability than Pt/C in a zinc–air battery.
It seems that ZrN is an outstanding co-catalyst because it combines excellent features of low cost, high activity and superior stability, benefiting in promoting the large-scale applications of electrochemical energy conversion.
Morover, this work can shed light on the further investigations of cost-effective and efficient nitride catalysts, and pave the way for the extensive use of clean energy.
This study was supported by Natural Science Foundation of China (No. 21471147), and National Key Research and Development Plan (No. 2016YFB0101205).
Most electrochemical energy devices, like fuel cells and metal-air batteries, need catalysts to boost power at acceptable rates. Oxygen reduction reaction (ORR) is one of the most fundamental reactions involved in electrochemical energy devices, which has greatly influenced the power delivery from devices.
Pt is currently the most widely used and commercial ORR catalyst. However, it is a scarcely available metal (37 ppb in Earth’s crust) with high cost (US$28.3 g-1 as the 2018 average price), which limits the large-scale applications of electrochemical energy devices.
To address this issue, the reaesrch group used a urea–glass route at moderate temperatures to prepare ZrN nanoparticles (NPs), which could substitute and even surpass the high-cost Pt as a catalyst for oxygen reduction under alkaline conditions.
As-synthesized ZrN NPs showed a high oxygen reduction performance with the same activity as that of the traditional Pt-on-carbon (Pt/C) commercial catalyst. Furthermore, in 0.1 M KOH solutions, both materials showed the same halfwave potential (E1/2 = 0.80 V) and ZrN exhibited a higher stability (ΔE1/2 = -3 mV) than the Pt/C catalyst (ΔE1/2 = -39 mV) after 1,000 ORR cycles. Besides, ZrN delivered a greater power density and cyclability than Pt/C in a zinc–air battery.
It seems that ZrN is an outstanding co-catalyst because it combines excellent features of low cost, high activity and superior stability, benefiting in promoting the large-scale applications of electrochemical energy conversion.
Morover, this work can shed light on the further investigations of cost-effective and efficient nitride catalysts, and pave the way for the extensive use of clean energy.
This study was supported by Natural Science Foundation of China (No. 21471147), and National Key Research and Development Plan (No. 2016YFB0101205).
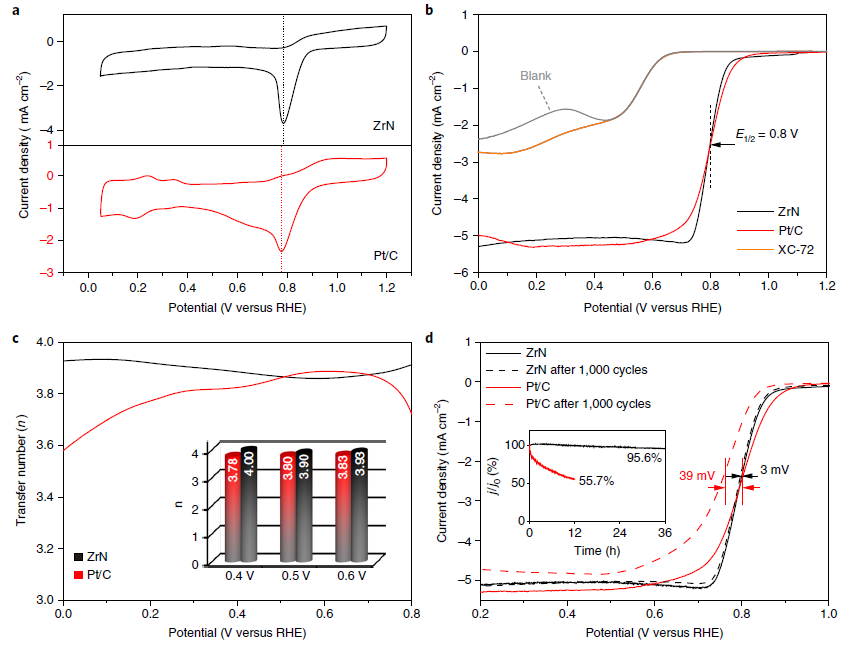
Fig. ORR catalysis properties of nanoparticulate ZrN and Pt/C in an O2-saturated 0.1 M KOH solution (Image by NIMTE)
Contact
HUANG Ye
Ningbo Institute of Materials Technology and Engineering
E-mail: huangye@nimte.ac.cn
