Prof. CHEN Liang’s research group at the Institute of New Energy Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences (CAS) prepared an iridium-free and low-ruthenium-content oxide (Cr0.6Ru0.4O2) derived from a metal-organic framework. The substance is quite active and low-cost, featuring excellent oxygen evolution reaction performance in acidic conditions with a record low overpotential of 178 mV at 10 mA cm-2. This study was published in Nature Communications.
Renewable energy sources will be of great significance in the near future. However, such resources are usually intermittent. For instance, solar energy is not available all day long due to weather changes and earth rotation. Thus it is quite necessary to explore the technology for converting the surplus power obtained from renewable energy sources to storable energy carriers (such as hydrogen).
Members of Prof. CHEN’s group have committed themselves to developing efficient electrocatalysts for the oxygen evolution reaction (OER). Recently Dr. LIN Yichao prepared a new CrO2-RuO2 solid solution by using a Cr-based metal-organic framework (MOF) and determined the structure of the solid solution via PXRD characterizations and refinement. It was observed through the atomic-solution high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) that in this structure Cr and Ru atoms are evenly distributed in the same nano-monocrystal. The solid solution exhibited an overpotential of merely 178 mV at the current density of 10 mA cm-2, and the overpotential only increased by 11 mV after 10,000 times of circulation, much more superior to the commercial RuO2. Such excellent performance, according to Dr. LIN, is due to the shortening of Ru-O bond length and the valence of Ru atoms, which is slightly higher than +4, in the crystal structure caused by the strong electron withdrawing of Cr with a valence of +4. The reason for the superb performance was also verified theoretically. Dr. TIAN Ziqi found through density functional theory (DFT) calculations that the catalytic activity of Ru is increased because of the electron withdrawing of Cr with a valence of +4 in the lattice, thus reducing the reaction energy barrier.
It is worth noting that Ru only accounts for only 40% of the solid solution, thus the costs of this electrocatalyst could be significantly reduced, making it ideal for commercialization and industrial application.
This study was supported by the National Science Foundation of China (Nos. 51472255 and 51602320) and the Program of Innovative Teams of Ningbo Municipal 3315 Plan (No. 2015B11002).
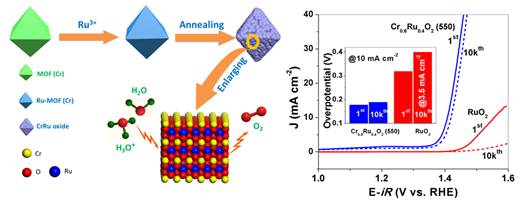
Schematic illustration of the preparation of CrO2-RuO2 solid solution for OER application in acidic conditions (left) and the experimental OER performance (right) (image by CHEN et al.)
(Courtesy of CHEN Liang, LIN Yichao and TIAN Ziqi)
Contact
XU Linqi
Ningbo Institute of Materials Technology and Engineering
E-mail: xulinqi@nimte.ac.cn

