Lithium (Li) metal has been widely considered as the most attractive anode material due to its ultrahigh theoretical capacity (3860 mA h g-1) and low negative redox potential (-3.040 V vs. the standard hydrogen electrode). Its practical application not only can significantly improve the energy densities of conventional batteries, but also expands the cathode candidates from the Li-containing ceramics to materials without lithium, such as oxides and fluorides.
To achieve practical application of Li metal electrodes, two fundamental challenges still need to be resolved: (1) accommodating the large change in electrode volume during cycling that creates significant mechanical instability and cracks in electrodes and their interfaces; (2) preventing the side reactions of Li metal towards the electrolyte that produce a solid electrolyte interphase (SEI) layer, which cannot withstand mechanical deformation and continuously break and repair during cycling, resulting in low Coulombic efficiency and short cycling life.
To address the harmful problematic of instable interfaces of Li metal anodes, the research group of Novel Electrochemical Energy-Storage Devices from Ningbo Institute of Materials Technology and Engineering has developed a series of protective structures to provide efficacious volume confinement of Li metal surfaces, with aim of stabilizing the SEI layers for high Coulombic efficiency and long lifespan of the Li metal anodes (Figure 1).
In the first protective model, we have fabricated a porous Al2O3 layer as an inorganic “host” to accommodate the deposited Li, and in parallel, a reinforced SEI was in-situ formed across this porous layer by using an electrolyte additive (Figure 1a). Based on its synergetic effect, such a composite structure has enabled a Coulombic efficiency of Li plating/stripping as high as 97.5-98% in carbonate-based electrolyte (J. Mater. Chem. A, 2016, 4, 2427-2432 ).
In the second protective model, we have synthesized a 3D porous Cu current collector with in-situ formed Li2O as reinforcing agent (Figure 1b). On one hand, the 3D Cu structure could efficiently reduce the local current density with aim of hindering the growth of Li dendrite; on the other hand, the Li2O reinforcing agent could stabilize the structure by extending the cycling life of Li anodes for more than 150 cycles (ACS Appl. Mater. Interfaces 2016, 8, 26801-26808 ).
In the third protective model, we have realized the formation of 3D SEI layer across a carbonaceous porous structure (Figure 1c). Thanks to the rigid carbonaceous structure with related 3D SEI layer coating, the Li plating/stripping efficiency was higher than 98% for more than 300 cycles (J. Mater. Chem. A, 2017, 5, 9339-9349 ).
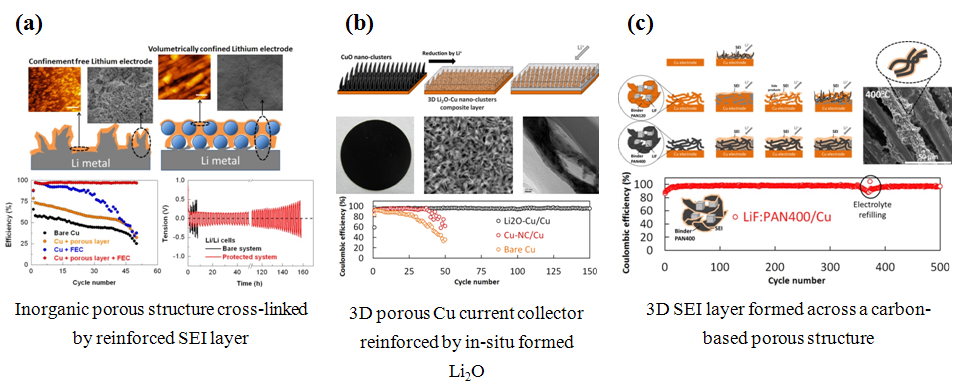
Figure 1. Surface protective structures for Li metal protection.
Recently, our group has cooperated with the teams of Prof. Xiangxin Guo from Shanghai Institute of Ceramics, Chinese Academy of Sciences, and Prof. Ji-Guang (Jason) Zhang from Pacific Northwest National Laboratory, (PNNL), to develop a transplantable protective layer cross-linked by nanoscale LiF domains for Li metal anodes (Figure 2). Such a Transplantable LiF-rich Layer (TLL) could isolate the deposited Li metal from the electrolyte, and thus improve significantly the cycling stability of Li metal anodes. With TLL protection, Cu-Li cells could stably operate for more than 300 cycles with a CE of ~ 98%. In a Li-LiFePO4 system, after cycling 1000 cycles, namely 6-month operation, the electrode protected by a TLL still retains its shiny metal surface (Nano Energy 2017, 39, 662-672 ). Our results demonstrate that the TLL could be an effective strategy to stabilize the Li/electrolyte interface for stable Li metal battery systems.
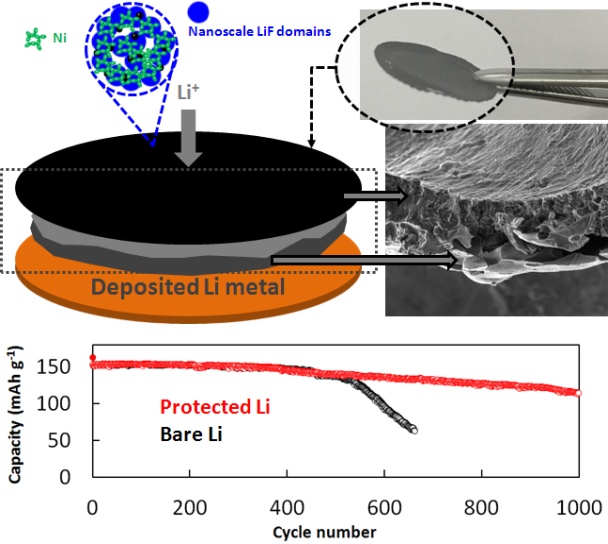
Figure 2. A transplantable protective layer based on nanoscale LiF domains for Li metal protection.
The authors gratefully acknowledge the financial support from the China Postdoctoral Science Foundation funded project (Grant No. 2015M570530 and 2016T90556), Ningbo Natural Science Foundation (Grant No. 2016A610278), Zhejiang Provincial Natural Science Foundation of China (Grant No. Q17E020023), and National Natural Science Foundation of China (NSFC) for the research fund for International Young Scientists (Grant No. 51650110490).
Prof. Deyu WANG: wangdy@nimte.ac.cn
Associate Prof. Zhe PENG: pengzhe@nimte.ac.cn
All Images by ![]()

