5-Hydroxymethylfurfural (HMF), an important platform chemical which can be derived from lignocellulosic carbohydrates, has been widely used for the production of a number of valuable derivatives, such as 2, 5-dimethylfuran, 1, 6-hexanediol, and levulinic acid.
Currently, HMF can be directly produced through acid-catalyzed dehydration of carbohydrates (including fructose, glucose, and cellulose). Among these chemicals, catalytic dehydration of fructose to HMF has been the most extensively investigated in different solvent systems. Although water is the most environment-friendly and abundant solvent, the selectivity of HMF is very low when using pure water as the reaction medium for dehydration of fructose. Other solvents, such as DMSO, ionic liquids and biphasic solvents etc., have been chosen to improve the selectivity of HMF. Ionic liquids, which are organic salts with low melting points and low toxicity, have therefore been emerged as an environmentally benign solvent. Hence, using ionic liquids as solvent for the dehydration of fructose to HMF have attracted a lot of attentions.
Zeolites, as one of the most representative heterogeneous catalysts, have frequently been studied in the dehydration of fructose to HMF recently. According to the literatures, the selectivity of HMF was strongly depending on the pore structure and acidity of the zeolites. Although many efforts have been devoted to improve the HMF yield, the performances of the zeolite catalysts are still unsatisfactory. We notice that most of the zeolites (including Beta, ZSM-5, Y, and mordenite) tested in the literatures possess multidimensional channel systems and large amount of strong acid sites, which might introduce the side reactions and reduce the yield of HMF.
Zeolite L owns a one-dimensional 12-ring undulating channels with pore opening of 0.71 nm in diameter, and has been widely used in many hydrocarbon conversion reactions. Typically, the parent zeolite L is a non-acidic zeolite. However, its acidity could increase by NH4-exchange post-treatment. Recently, a cooperation of Jian ZHANG’s group and Yajie ZHANG’s group from Ningbo Institute of Materials Technology and Engineering, CAS, have successfully developed an acidic zeolite L for efficient dehydration of fructose to 5-hydroxymethylfurfural in ionic liquid. As shown in Figure 1a and 1b, the NH4-exchange post-treatment effectively increased the acidity of zeolite L. 99.1% yield of HMF was achieved from the dehydration of fructose by using the KL-80°C-1h sample (KL zeolite treated with 1M NH4NO3 solution at 80°C for 1h). The high efficiency could be attributed to the appropriate strength of Br?nsted acid sites and the less amounts of Lewis acid sites, which facilitated the dehydration of fructose and suppressed the condensation of fructose into humins, respectively. The catalyst could be reused for four recycles without obvious decrease in the activity, indicating a great potential of industrial applications of zeolite L for the HMF production.
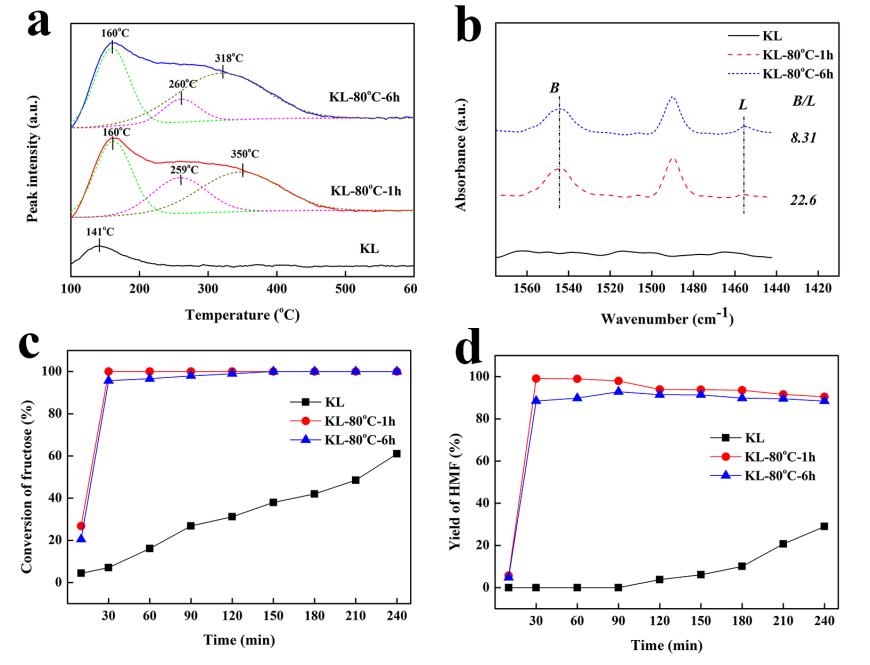
Figure 1. NH3-TPD profiles (a), pyridines FT-IR spectra (b), fructose conversion (c) and HMF yield (d) of samples.
B: Br?nsted acid, L: Lewis acidity. Reaction conditions: 10 g [bmim]Br, 1 g fructose, 0.1 g catalyst, 120°C.
(Image by NIMTE)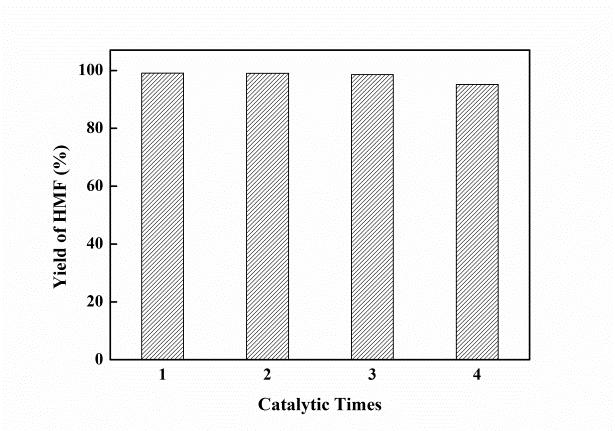
Figure 2. The yield of HMF on KL-80oC-1h catalyst in cycle usage test.
(Image by NIMTE)
The above results have been published on ChemSusChem, 2017, 10(8): 1669–1674.
The authors are grateful for the financial support from the National Natural Science Foundation of China (No. 51422212 and 21307142), Key Projects of the National Science and Technology Pillar Program (No. 2015BAD15B08), Key Research Program of Frontier Sciences of CAS (No. QYZDB-SSW-JSC037) and Ningbo Science and Technology Bureau (No. 2015A610248, 2015B11003 and 2014D10004)
Article links (http://onlinelibrary.wiley.com/doi/10.1002/cssc.201700239/epdf).
Dr. Hualei HU: huhualei@nimte.ac.cn
All Images by ![]()

