Catalytic CO oxidation has been extensively investigated and applied in many fields, such as air purification, automotive exhaust cleaning, CO removal of cigarette smoke, and CO2 lasers.
However, under the real condition, the presence of water vapor inevitably leads to catalyst deactivation through temporary poisoning of the exposed active sites. Noble-metal catalysts such as Pd, Pt, and Au have been widely recognized as the preferred choices, but both high costs and finite resources limit their application. Alternatively, a series of cheap transition-metal oxides and their composites have drawn tremendous attention. Among them, cryptomelane-type octahedral molecular sieve (K-OMS-2) is one of the most attractive materials. Nevertheless, the activity of K-OMS-2 for CO oxidation is still unsatisfactory, especially under wet reaction.
To solve this problem, Professor Jian Zhang and his group in Ningbo Institute of Materials Technology and Engineering, CAS have successfully introduced secondary metal ions (Co3+) into the framework sites of K-OMS-2 to enhance the catalytic activity for CO oxidation under real wet conditions.
The incorporation of Co induced a morphology change in K-OMS-2 from nanorods to nanofibers and an increase in the specific surface area from 70.6 to 188.3 m2 g-1 (Fig 1). Co-doped K-OMS-2 was shown to be able to completely convert CO at 100 oC in the presence of approximately 3% water vapor, giving a reaction rate 17.4 times that of the undoped K-OMS-2 (Fig 2). They also compared in detail the catalytic performances of the reported catalysts under wet condition. Co-doped K-OMS-2 display a T50 value (temperature at 50% conversion) similar to noble-metal catalysts, including Au/CeO2, Pt/CeO2, and Au1Cu1/CeO2, under wet conditions, and its reaction rate at T50 is comparable to that of the same noble-metal catalysts. The promotion effect of Co on the catalytic activity is believed to originate from the enhanced redox capacity and surface area of K-OMS-2, whereas the improved surface hydrophobicity may induce superior water-tolerance performance.
This work has been published in the form of a cover article on ChemCatChem, 2017 (DOI:10.1002/cctc.201601681, Fig 3). It might provide a simple and promising route to synthesize cheap and water-tolerant catalysts for industrial applications in CO removal under moisture-rich conditions.
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (51422212, 21403261 and 21307142) and Zhejiang Provincial Natural Science Foundation of China (LR16B030001).
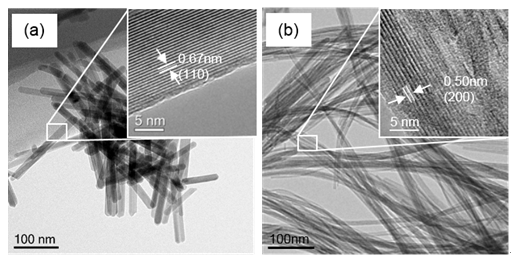
Fig.1. Morphological characterization. (a, b) HRTEM images of undoped and Co-doped K-OMS-2 catalysts.
(Image by NIMTE)
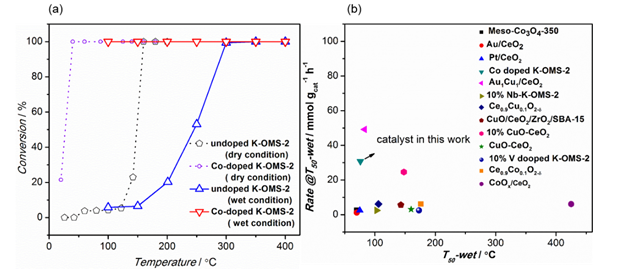
Fig.2. (a) % CO conversion as a function of temperatures over undoped and Co-doped K-OMS-2 catalysts: the effect of 3% water vapor. (b) Comparison of reaction rate at T50 (temperature at 50% conversion) with different catalysts reported.
(Image by NIMTE)

Fig 3. The back cover picture on ChemCatChem, 2017, 9: Cobalt-Doped K-OMS-2 Nanofibers: A Novel and Efficient Water-Tolerant Catalyst for the Oxidation of Carbon Monoxide
Pro. Dr. Lei WANG: wanglei@nimte.ac.cn
Jie YANG: yangjie@nimte.ac.cn
All Images by ![]()

