Membrane catalysis reaction which was developed recently is a promising and novel catalytic reaction technology with wide application, and it can synchronously fulfill two processes of catalysis reaction and production separation, thus achieving the integrative processes of catalysis-reaction-separation. Membrane catalysis reaction has many advantages, such as process simplify, cost save and energy reduce.In the past two decades, attributing to process intensification by combining catalysis and molecular sieving in one apparatus, zeolite-based catalytic membrane reactors (CMRs) have drawn increasing interest for the selective in-situ removal of one or more products during a reaction to overcomean equilibrium limitation and thus increasing the conversion.
Dimethyl ether (DME) is a promising and clean alternative to diesel fuel due to its high cetane number, low auto-ignition temperature, and low emission of pollutants. Furthermore, DME is also an important feedstock for producing many platform chemicals such as lower olefins, methyl acetate, formaldehyde, and dimethyl sulfate. So far, two main synthetic routes have been developed to produce DME, that is, methanol dehydration and syngas conversion in a two-step process with methanol as an intermediate.Conventionally, the synthesis of DME by methanol dehydration is regarded as the most mature route and widely used in the chemical industries. However, methanol dehydration to DME is equilibrium-controlled reaction.
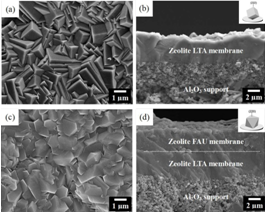
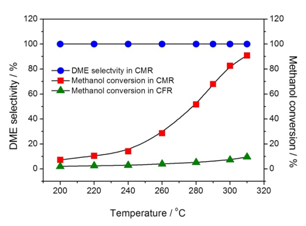
Considering the reaction characteristics of DME synthesis from methanol, Prof. Dr. Aisheng Huang and his group in Ningbo Institute of Materials Technology and Engineering, CAS have successfully developed a novel bifunctional zeolite catalytic membrane reactor (CMR) for methanol dehydration to DME (Figure 1). The CMR has a sandwich structure: top membrane (zeolite FAU) acts as the catalyst of methanol dehydration to DME and the other membrane (zeolite LTA) acts as the separator for selective removal of the produced water in the reaction system. The sandwiched zeolite FAU-LTA membrane (Figure 2) was prepared on porous α-Al2O3 tube by using 3-aminopropyltriethoxysilane (APTES) as covalent linker between zeolite layer and α-Al2O3 support with a thickness of about 4.0 μm for LTA and 3.5 μm for FAU. Figure 3 shows the catalytic performance of the zeolite catalytic membrane reactor towards methanol dehydration to DME.The performance of zeolite H-FAU powder innomethal dehydration to DME was also tested in a catalytic fixed-bed reactor (CFR) as a reference. As shown in Figure 3, the conversion of methanol into DME isrelatively low in the CFR, and the maximum conversion of methanol into DME was only 9.5% at 310 ℃. On the contrary, the conversion of methanol into DME was significantly enhanced for the CMR, while the maximum methanol conversion reached 90.9% at 310 ℃, which is much higher than that in the CFR (9.5%). Through the selective and continuous removal of the produced water from the reaction system with the hydrophilic zeolite Na-LTA membrane, the thermodynamic equilibrium limitation of methanol dehydration to DME is removed in the CMR, resulting in a great enhancement of methanol conversion. Furthermore, the stability of the catalyst is improved by the in situ removal of the produced water, which is also helpful to increase the selectivity of DME formation.
The above results have been published on Angewandte Chemie International Edition, 2016, 55(41): 12678-12682.
Figure 3.DME selectivity and conversion of methanol into DME as a function of the reaction temperature in the CMR and CFR (CMR: catalytic membrane reactor; CFR: fixed-bed reactor).
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 21276262), the External Cooperation Program of BIC, CAS (Grant No. 174433KYSB2013005), the National High Technology Research and Development Program of China(Grant No. 2015AA03A602),the Ningbo Science and Technology Innovation Team (Grant No. 2014B81004), the China Postdoctoral Science Foundation (Grant No. 2015M571907), and Ningbo Municipal Natural Science Foundation (Grant No. 2015A610055).
Prof. Dr. Aisheng Huang: huangaisheng@nimte.ac.cn
Dr. Chen Zhou: zhouchen@nimte.ac.cn
All images by