Lithium ion battery (LIBs) has been paid considerable attentions as the dominant power sources to meet the ever-growing demands of technologies ranging from portable electronic devices and electric vehicles to grid-scale energy storages. Consequently, safety issues of LIBs become a significant concern owning to the flammable and potentially dangerous liquid electrolytes, especially in large capacity applications such as electric vehicles and energy storage systems. Therefore, solid electrolytes is regarded the most promising solution to replace liquid electrolytes. Solid electrolytes present potential advantages, such as prevention of electrolyte leakage, high electrochemical stability, high thermal stability and absence of problems relating to vaporization of organic solvents, which could improve the safety substantially. Specially, organic/inorganic hybrid solid electrolytes have been attracted more and more attentions due to their flexible, good compatible between the interface of electrolyte and electrodes, and can be prepared in large scale by roll-to-roll fabrication processes.
Recently, Prof. Xiaoxiong Xu and his group in Ningbo Institute of Materials Technology and Engineering (CAS) have successfully developed a series of novel organic/inorganic hybrid solid electrolytes. Li10GeP2S12 (LGPS) was incorporated into polyethylene oxide (PEO) matrix to fabricate a novel hybrid solid electrolyte by the first time. The lithium ion conductivities of as-prepared electrolyte were evaluated, and the optimal hybrid solid electrolyte exhibited a maximum ionic conductivity of 1.18 × 10-5 S cm-1 at room temperature and an electrochemical window of 0 - 5.7 V. The LGPS microparticles, acting as active fillers incorporation into the PEO matrix, have a positive effect on the ionic conductivity, lithium ion transference number and electrochemical stability. The LiFePO4/Li battery using such hybrid solid electrolyte exhibits fascinating electrochemical performances in term of high capacity retention (92.5% after 50 cycles at 60 °C), as shown Figure 1. Furthermore, solid plasticizer SN was incorporated into the PEO/LGPS to further improve its conductivity. The maximum ionic conductivity of PEO/LGPS/SN is close to 10-4 S.cm-1 at 25 °C as shown in Figure 2. This new electrolyte presents good cycling and rate performance at 40 °C of the all-solid-state lithium battery, whereas, the cells that fabricated by similar electrolytes usually performed under 60 °C.
Because of LGPS is sensitive to the environment and difficult to produce, Li1.5Al0.5Ge1.5(PO4)3 (LAGP) is selected to incorporated into PEO matrix to fabricate PEO/LAGP hybrid solid electrolyte at ambient. The optimal electrolyte exhibits a maximum ionic conductivity of 6.76 × 10-4 S cm-1 and an electrochemical window of 0 - 5.3 V at 60 °C. This work provides a promising PEO/LAGP hybrid solid electrolyte prepared by a simple method which can be manufactured easily in industry scale.
In additional, a new solid electrolyte membrane with tunable network structure is successfully designed and prepared via a facile one-pot reaction. The network structure could be tuned by changing the molar ratio of TMPEG and NPEG or the molecular weight of NPEG. The optimal electrolyte presents a maximum conductivity of 1.10×10-4 S cm-1 under 30 °C, and an 18-fold ionic conductivity enhancement in that of PEO-based electrolyte. It also exhibits wide electrochemical window (0 - 5.4 V), excellent compatibility with metallic Li, and superior mechanical properties. The all-solid-state lithium batteries LiFePO4/Li are assembled with this electrolyte, and present good cycling and rate performance under 60 °C. The capacity retention ratio can be retained at 90.6% after 100 cycles at 0.2 C as shown in Figure 4. This new copolymer electrolyte may become a promising candidate for applications in all-solid-state lithium battery.
The results have been published on J. Power Sources, 2016, 301, 47-53;Electrochim. Acta, 2016, 210, 905-914; Solid State Ion., 2016, 295, 65-75; J. Power Sources, 2016, 331, 322-331. Three related Chinese patents have been filed (CN 201511033107.7; CN 201610662266.1; CN 201610696494.0).
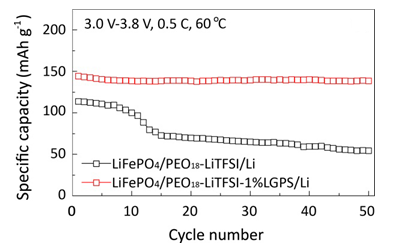
Figure 1. The capacity retentions of cell LiFePO4/Li at 0.5 C under 60 °C.
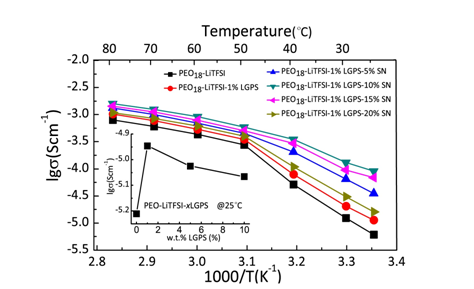
Figure 2. Arrhenius plots for the ionic conductivities of electrolyte membranes;

Figure 3. Photograph and SEM images of prepared solid electrolyte membrane;
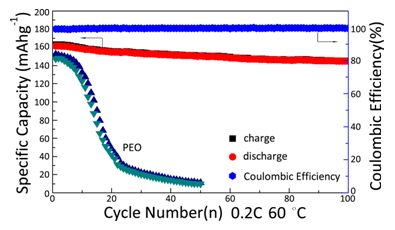
Figure 4. The capacity retentions of cell LiFePO4/Li at 0.2 C under 60 °C.
The authors are grateful for financial support from the National Natural Science Foundation of China (Grant No. 51502317), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA09010201), the Key Scientific and Technological Innovation Team Project of Zhejiang province (Grant No.2013PT16) and Natural Science Foundation of Ningbo (Grant No. 2015A610238).
Prof. Xiaoxiong Xu:xuxx@nimte.ac.cn
Dr. Shaojie Chen:chenshaojie@nimte.ac.cn
Research Group Url: http://english.nimte.cas.cn/pe/fas/201509/t20150911_152262.html
All Images by ![]()

