With the increased demand for clean energies such as solar and wind power that are integrated into utility grid, rechargeable aqueous metal-ion batteries (RAMB) have drawn a great deal of attention because of their better safety, higher rate capability, lower cost and more eco-friendliness relative to organic counterparts. To date, a variety of RAMB on a basis of metal-ion intercalation chemistry have been explored. However, most of them suffer the problem of low energy density due to the low voltage output (< 1.5 V). Seeking high-voltage RAMB as viable alternatives to commercial aqueous batteries for large-scale energy storage still remains a big challenge.
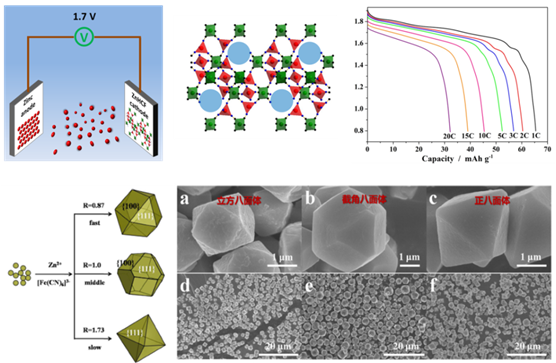
In 2013, a team led by Prof. Zhaoping Liu from Ningbo Institute of Materials Technology and Science, the Chinese Academy of Sciences, firstly proposed an innovative concept of M+/N+ mixed-ion electrolytes to construct two batteries Na0.44MnO2/TiP2O7 and LiMn2O4/Na0.22MnO2 (Scientific Reports 2013, 3, 1946). Unlike traditional “rocking-chair” lithium-ion battery, such batteries operate based on the immigration of dual shuttles between electrode and electrolytes. Their one important characteristic is that the total concentration of M+ and N+ is fixed during charging/discharging, but the M+/N+ ratio is changed. Unfortunately, all of them are still limited to low-voltage output as well as rapid capacity fading, which greatly hinder their practical application. Then, the team continued to develop another two batteries based on M+/N+ mixed-ion electrolytes: Ni1Zn1HCF/TiP2O7 and Ni1Zn1HCF/NaTi2(PO4)3 (ChemSusChem 2014, 7, 2295). Their voltage outputs exceed 1.2 V, and they are made of low-cost electrode materials with three dimensional framework structures, which are promising for the application in the field of large-scale energy storage. In addition, the team has discovered that rhombohedral zinc hexacyanoferrates (ZnHCF) can behave good hosts for divalent ion Zn2+. By employing it as a cathode material, a unique zinc ion battery Zn/ZnHCF with a voltage output of 1.7 V is obtained. They have also developed a simple method to prepare ZnHCF with cubooctahedral, truncated octahedral and octahedral shapes. Moreover, a morphology-dependent electrochemical performance of ZnHCF is found: cubooctahedral > truncated octahedral > octahedral. These results have been published in Advanced Energy Materials (2015, 5, 1400930) and Scientific Reports (2015, 5, 18263).
Very recently, the team has introduced open-framework indium hexacyanoferrate (InHCF) as a cathode material for RAMB. By combining it with carbon-coated TiP2O7 and NaTi2(PO4)3 as anode materials, a series of RAMB based on M+/N+ mixed-ion electrolytes with voltage output > 1.2 V are demonstrated. Among them, InHCF/NaTi2(PO4)3 battery exhibits a specific energy of 56 Wh kg-1 with a voltage output of 1.6 V, and InHCF/TiP2O7 battery can deliver a specific power of 2700 W kg-1 that is comparable to that of an ultra-capacitor. Finally, water-mediated cation intercalation in InHCF is firstly revealed by using multiple characterization means coupled with density functional theory (DFT) calculations. Water is supposed to be co-inserted with Li+ or Na+, which evidently raises the intercalation voltage and reduces diffusion kinetics. As for K+, water is not involved in the intercalation because of the channel space limitation in InHCF. The relevant results are published as an article in Nature Communications (2016, 7, 11982).
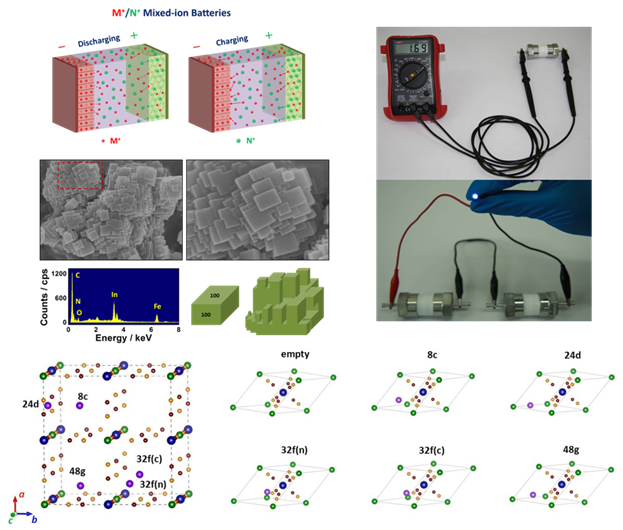
Above work are financially supported by National Natural Science Foundation of China (grant no. 51404233), Key Research Program of Chinese Academy of Sciences (grant no. KGZD-EW-T08-2), Natural Science Foundation of Zhejiang Province and Ningbo city (grant nos LY15B030004 and 2014A610044).
Prof. Liang Chen: cl@nimte.ac.cn
Research Gruop Url : http://english.nimte.cas.cn/rh/rd/newenergy/battery/batresearch/
All Images by ![]()

