Solid electrolyte interface (SEI), a sacrificial layer reduced from electrolyte solution, plays a vital role in operation, safety and cyclability of lithium ion-batteries (LIBs).It determines not only the reversibility of Li+ intercalation at the anode but also strongly affects the kinetics of Li+ transport across the electrode-electrolyte interface.Much research efforts are currently directed at understanding the morphological evolution of SEI to improve LIB performance rate. To date, details on SEI formation (3D evolution), composition, stability, and its influence on the performance of LIBs are still controversial.
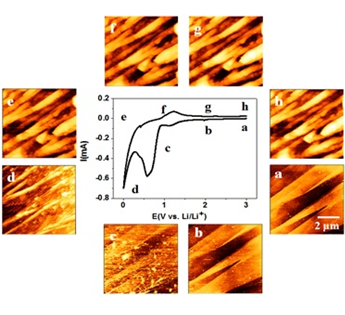
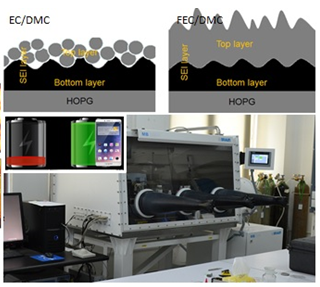
Dr Cai Shen and his research team, Ningbo Institute of Materials Technology & Engineering, Chinese Academy of Sciences has made significant progress in the understanding of the SEI morphological evolution by using a combination of in-situ electrochemical AFM and ex situ XPS.By using the electrochemical atomic force microscopy (EC-AFM), Dr Shen and his team has successfully presented a real-time views of SEI morphological evolution. Complemented by an ex situ XPS analysis, fundamental differences of SEI formation from ethylene carbonate (EC) and fluoroethylene carbonate (FEC)-based electrolytes during first lithiation/delithiation cycle on HOPG electrode surface were revealed. SEI layer formed by reductive decomposition of the FEC/DMC electrolyte was mainly composed of LiF and trace amount of Li2CO3. The SEI layer was dense and hard which can protect graphite against dendrite formation and potentially improve cyclability of LIBs.
Unlike traditional electrolytes evaluation which involves complex and tedious procedures, a combination of in-situ electrochemical AFM and ex situ XPS serves as a fast diagnostic tool to evaluate the properties and quality of SEI formed on different electrodes from diverse electrolytes and additives.
The aforementioned findings is published in ACS Applied Materials & Interfaces.
Dr Cai Shen: shencai@nimte.ac.cn
All Images by ![]()

