Carbon nanomaterials feature high chemical compatibility. Surface functionalization endows these materials excellent catalytic activities in several reactions such as alkane activation, biomass conversion and electrochemical oxygen reduction. However, the functionalization could produce a wide variety of functionalities and it is difficult to quantify them. As a result, the structure-behavior relationships in the carbon catalyzed reactions are not clear yet.
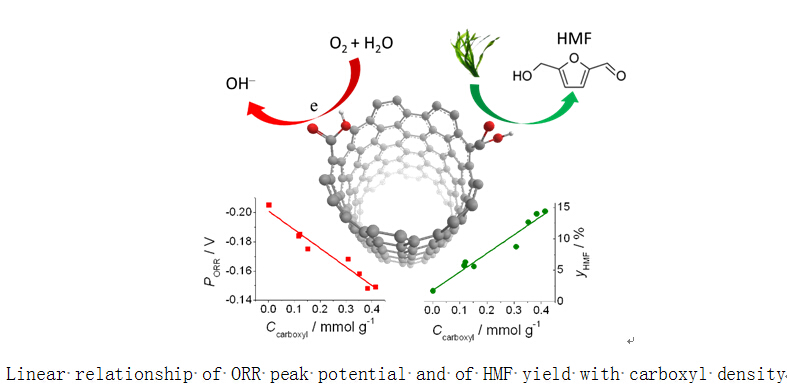
Recently, a cooperation of Jian Zhang’s group and Yajie Zhang’s group from Ningbo Institute of Materials Technology and Engineering, CAS made a new progress in carbon catalyzed reaction mechanism.They found a pivotal role of carboxyl group on the surface of carbon nanotubes (CNTs) in the catalytic reactions of electrochemical oxygen reduction reaction (ORR) and agar conversion to 5-hydroxymethylfurfural (HMF). They employed an environment-friendly ozone oxidation craft self-developed (Patent appl. No. CN201310686814.0) to functionalize a commercial CNTs by introducing some functionalities of carboxyl, phenol and lactone. These functionalities were quantified by using an automatic Boehm titrator self-developed (Computer software copyright No.2014SR030807). The correlations between the catalytic behaviors and the functionalities distribution revealed that the ORR peak potential and the HMF yield were both in the linear relationship with the carboxyl density. The finding provides an important inspiration for the above two reactions, i.e. developing the functionalization with unique carboxyl group for carbon nanomaterials.
This work has been published on Nano Research (IF=6.963, DOI: 10.1007/s12274-014-0660-3) sponsored by Springer.
Prof. Jian Zhang jzhang@nimte.ac.cn, Dr. Yexin Zhang zhangyexin@nimte.ac.cn
Research Group Url: http://english.nimte.cas.cn/rh/rd/newmaterials/Nanocarbon/nanmembers/201204/t20120410_83658.html
All Images by![]()

