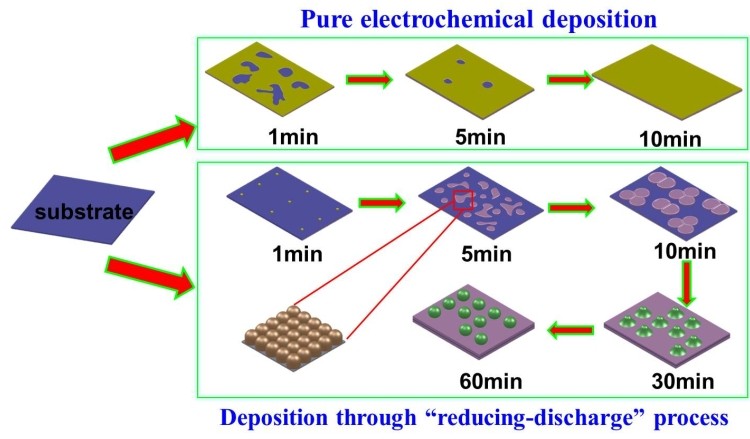
The wetting behavior of solid surfaces by a liquid is a very important aspect of surface chemistry. The superhydrophobic solid surfaces have many practical applications like self-cleaning, anticorrosion, micro-droplet transportation, oil-water separation; also super hydrophobic solid surfaces can be used in patterned click chemistry. In 2013, Prof. Xuedong Wu’s Group at NIMTE prepared Patterned tailored hydrophobic films designed by synergy effect of electrochemical deposition and chemical deposition. The film morphology changes and the roughness of film surfaces gradually become bigger with the increased solution temperature. The gradually increased film roughness leads to the film’s hydrophobicity changing from hydrophobic to superhydrophobic.
Recently, an overall research has been made to intensively investigate the growth mechanism of these Ni-P alloy patterned films. The experiment of Ni-P alloy films deposited at different time has been performed. Herein, the formation mechanism of this superhydrophobic surface is explained in a more acceptable way. The different stages of film growth observed though SEM images and XPS result evidencing the occurrence of electrochemical deposition strongly support the hypothesis of the synergistic effect of electrochemical deposition and chemical deposition. It is the addition of reducing agent to the solution that induces the formation of nano scale particles appearing at the surface morphology. The multiscale surface features are formed by the combination of the theory on the unstable growth process. The mastoid-like structures in different growth stages are evidence that these films are grown based on the progressive nucleation of Scharifker-Hills rule.
According to this rule, two diagnostic relationships can be used to determine whether the nucleation of the Ni-P patterned alloy films is instantaneous or progressive. There are large and small protrusions, which mean the formation of new nuclei is faster than the growth of existing nuclei. The result is the progressive superposition of the currents of formation and growth of each nucleus. The surfaces of these protrusions at different growth stages show different surface smooth degrees which can be explained by the unstable growth process. The immature ones hide under the patterned films, and the diffusion layer is thick here, making the metal ions difficult diffusing into these protrusions’ surfaces. However, when the protrusions grow larger, they can stretch into the bulk solution, and the diffusion layer here is thin. Following the unstable growth theory, the thinner the diffusion layer becomes, the smaller the particles tend to appear. The particles size on immature protrusions is large making the protrusions appear rough. On the contrary, the mature ones appear smooth. The existence of phosphite radicals (H2PO3-) can be certified by XPS data. This form of element P gives the direct evidence for the occurrence of chemical deposition.
The alloy films growth by Reducing-discharging process can give a more clear direction for the future work in the research.
The research results have been published in ACS Applied Materials & Interfaces, 2014, 6 (2), pp 1053–1060
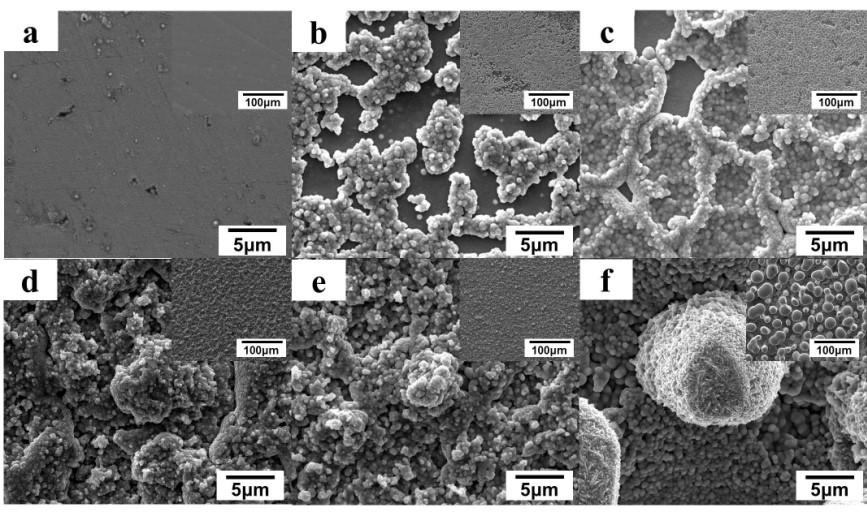
Figure 1. Effect of deposition time on the Ni-P alloy films surface morphologies. SEM images of Ni-P alloy films deposited for a) 1 min; b) 5 min; c) 10 min; d) 20 min; e) 30 min; f) 60 min. the insets are low magnification SEM images.
|
Figure 2. A brief diagram of the mechanism of formation of pure Ni films (above) and Ni-P alloy pat-terned films (bottom). Prof.Xuedong Wu xdwu@nimte.ac.cn All images by
|