The wetting behavior of solid surfaces by a liquid is a very important aspect of surface chemistry. For a solid substrate, when a contact angle (CA) of water on it is larger than 150°, it is called superhydrophobic. On the other hand, when the CA of water on a surface is almost 0°, it is called superhydrophilic. The superhydrophobic solid surfaces have many practical applications like self-cleaning, anticorrosion, micro-droplet transportation, oil-water seperation; also superhydrophobic solid surfaces can be used in patterned click chemistry. Up to now, there have been many methods in preparing superhodryphobic solid surfaces, like sol-gel, self-assembling and electrodeposition and so on. Among them, electrochemical methods are widely used to form rough structures for their easy operation and wide application.
Recently, Prof. Wu’s Group at NIMTE prepared patterned tailored hydrophobic films designed by synergy effect of electrochemical deposition and chemical deposition. The films morphology changes and the roughness of film surfaces gradually become bigger with the increased solution temperature. The gradually increased film roughness leads to the film’s hydrophobicity changing from hydrophobic to superhydrophbic.
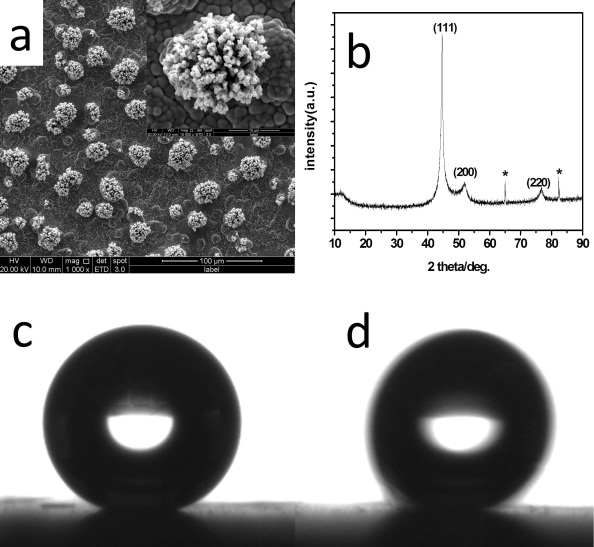 |
| Fig. left: (a) SEM images (inset is the larger magnification) and (b) XRD spectrum of Ni-P patterned film deposited at 80℃. (c) Optical images of water droplet on patterned films and (d) photograph of water droplet when it moves. Right: Typical SEM images of the Ni-P alloy films prepared at different temperatures. Deposition temperature is a ) 65℃; b) 70℃; c) 75℃; d) 80℃. These films show tailored hydrophobic property. |
Their research demonstrates that the synergy effect of the two deposition methods can dramatically change films morphology. The synergy means the simultaneous occurrence of the two depositions at the same condition. In chemical deposition, Ni2+ is reduced by reducing agent like H2PO2-. However, in the progress of electrodepositing Ni, the Ni2+ is being reduced by the electrons on cathode in the circuits. If reducing agent is added into the electrodeposition solution, the transition state of electrodepositing Ni will encounter the reducing agent, making the chemical deposition take place. The chemical deposition reaction can be significantly affected by the solution temperature. The higher the solution temperature is, the higher the reaction activity will be. The higher reaction activity makes the deposition progress uneven. Consequently, the film roughness deposited at the cathode is gradually changed with the solution temperature.
The research results have been published in Chem. Commun., 2013, 49 (24), 2424- 2426. The systematic research about the mechanism of synergy effect and the regulation of films morphology variation is in progress in their lab.
Prof. Xuedong Wu xdwu@nimte.ac.cn
Image by ![]()

