The size of magnetic nanopaprticles (NPs) has an important effect on their own physical properties, such as Curie temperature, coercivity, saturation magnetization, and their magnetization behavior as well. Therefore, the particle size is one of the important parameters of magnetic nanoparticles. There are specific requirements of particle size in their related application field, for instance, the accurate size requirement of soft and hard magnetic phase in two-phase nanocrystalline composite magnets. For a long time, it is a cutting-edge research topic for the control of small size of magnetic NPs and their response to bio-molecule. A great deal of attention has been given to magnetic NPs, especially for those with particle size in the range of 2-20 nm. Consequently, to prepare magnetic NPs with accurately controlled size using a simple and tunable method is one of the preconditions for their actual application.
Among the reported results, the size control of magnetic NPs was realized mainly by changing the ratio of surfactant and the metal precursors, or by adjusting the preparation process, which for example, changing the heating rate and so on to tune the nucleation and growth rate of magnetic NPs when thermal decomposition method was applied. However, usually many technology parameters need to be changed for these control methods as mentioned above which are not good to be widely applied. Besides, the obtainable size control of magnetic NPs is above 1-2nm, while for particle size control that is smaller than 1 nm, it is still not realized because of being out of control of decomposition rate of metal precursors. It is a necessary premise to clarify the growth mechanism, and then to realize the accurate size control of NPs. Currently, it is still a deficiency in understanding the growth mechanism of FePt NPs, and the main reason was ascribed to their fast rate of nucleation and growth.
Prof. Juan Du and her colleagues from the Nanoscale Magnetic Materials Group, the Ningbo Institute of Materials Technology & Engineering (NIMTE), Chinese Academy of Sciences (CAS) have investigated the growth mechanism and size control of FePt NPs for years.In their work, the decomposition rate of Fe precursor was effectively controlled via one intermediate, namely a complex Fe(CO)x-OAm formed by reaction between iron pentacarbonyl (Fe(CO)5) and oleyl amine (OAm, C18H35NH2), and also, the final results showed that the precursor prepared by Fe(CO)x-OAm and platinum(II) acetylacetonate (Pt(acac)2)can well control the nucleation and growth rate of FePt NPs. The transmission Electron Microscope (TEM) was applied to observe the whole process.
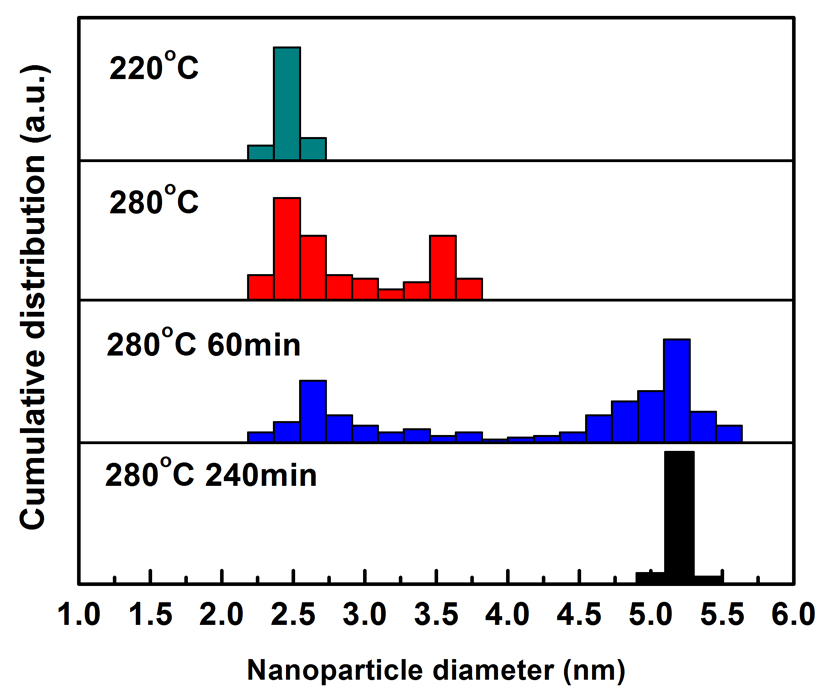
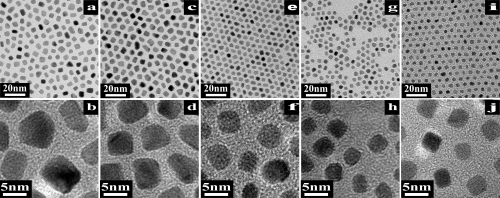
Their research showed that reaction temperatures have an intensive effect on the nucleation and growth process of FePt NPs during the synthesis process of FePt NPs. The growth process of FePt NPs obtained by controlling the complex temperature between OAm and Fe (CO) 5 was given in Fig.1 and Fig.2. The growth mechanism of FePt NPs was jointly controlled by Ostwald-ripening (OR) growth mechanism and oriented-attachment (OA) mechanism. The growth schematic of FePt NPs was shown in Fig.3. The ratio of OAm and Fe (CO) 5 can effectively suppress the OA process, and then can control the size and morphology of FePt NPs. FePt NPs with 0.5 nm size can be well controlled by controlling the complex temperature and ratio of OAm and Fe (CO) 5. The size of FePt NPs was 5.1, 4.7, 4.3, 4.0 and 3.6 nm respectively, and they have a very small size distribution and quasi- round shape, as shown in Fig. 4.
 |
|
Fig. 1 TEM images of FePt NPs synthesized from Fe(CO)x-OAm (prepared with OAm : Fe(CO)5 = 1 : 1 at 30 oC) at different stages during synthesis: when the temperature reached up to 220 oC (a), 280 oC (b) and refluxed at 280 oC for 60 min (c) and 240 min (d). |
 |
|
Fig. 2 Size distribution of FePt NPs obtained at different temperatures (220 oC and 280 oC) and different refluxing times (60 min. and 240 min. at 280 oC). |
 |
 |
| Fig. 3 Left: Proposed stages of FePt NP formation process: (i) rapid formation of a large number of FePt NPs nuclei; (ii) subsequent growth of larger particles, here both OA and OR happen; (iii) slow growth of particles at expense of smaller clusters via an OR process. (The dots represent the atomic monomer, and the circles with stripes represent the particles.) | Right: Two growth process of OR and OA nucleation and growth process captured by TEM and the final morphology of quasi-round and nano-rod shape FePt NPs. |
 |
|
Fig. 4 TEM images of FePt NPs with a mean size of (a) 5.1 nm; (c) 4.7 nm; (e) 4.3 nm; (g) 4.0 nm and (i) 3.6 nm synthesized from Fe(CO)x-OAm with molecular ratios of OAm: Fe(CO)5 of 1 : 1, 2 : 1, 3 : 1, 4 : 1 and 5 : 1, respectively. (b), (d), (f), (h) and (j) are respectively the magnified images of (a), (c), (e), (g) and (i). |
A possible route was provided in this work for investigating the growth mechanism of other NPs, i.e. by using intermediate to control the nucleation and growth rate, and then to observe the mechanism of NPs under in situ TEM observation, which can also be used as a beneficial reference to control the size and morphology of NPs in other systems prepared by chemical methods. Up to now, a related Chinese patent has been filed (201210207877.9). The related work has been published on Nanoscale, 5, 2454 (2013).
Professor Juan Du Dujuan@nimte.ac.cn
Research Group Url: http://english.nimte.cas.cn/rh/rd/nmm
All Images by ![]()

