Nanoionics is the study and application of phenomena, properties, effects and mechanisms of processes connected with fast ion transport in all-solid-state nanoscale systems. Many devices including lithium (Li)-ion battery, fuel cell, supercapacitor as well as the new emerging ionic memory are all closely related to this topic. Present interests in nanoionics are focused on the study of ions transportation and related properties in oxides, ion conductors and the heterostructure interfaces. To study ion transportation at the nanoscale and reveal related mechanisms can benefit the development of devices based on nanoionics, and also the exploration of novel physical phenomena at the nanoscale.
A group led by Prof. Runwei Li from the Ningbo Institute of Materials Technology & Engineering (NIMTE), the Chinese Academy Of Sciences (CAS) has been doing research in the nanoscale ion electrical transportation for years and achieved a series of progress. The researchers fabricated Nb/ZnO/Pt and ITO/ZnO/ITO thin film sandwiched structures through controlling the diffusion of niobium and oxygen ions at the nanoscale. They obtained quantum point contact structures at the atomic scale and observed quantum conductance at room temperature. (Adv. Mater., 24, 3941-3946 (2012) http://onlinelibrary.wiley.com/doi/10.1002/adma.201201506/pdf)
The group members also studied the diffusion of proton acid ions and their doping effects in polyazomethine by controlling electrical fields. They then obtained stable resistive switching devices which proved with excellent performances. (JACS, 134, 17408-17411 (2012) http://pubs.acs.org/doi/abs/10.1021/ja307933t)
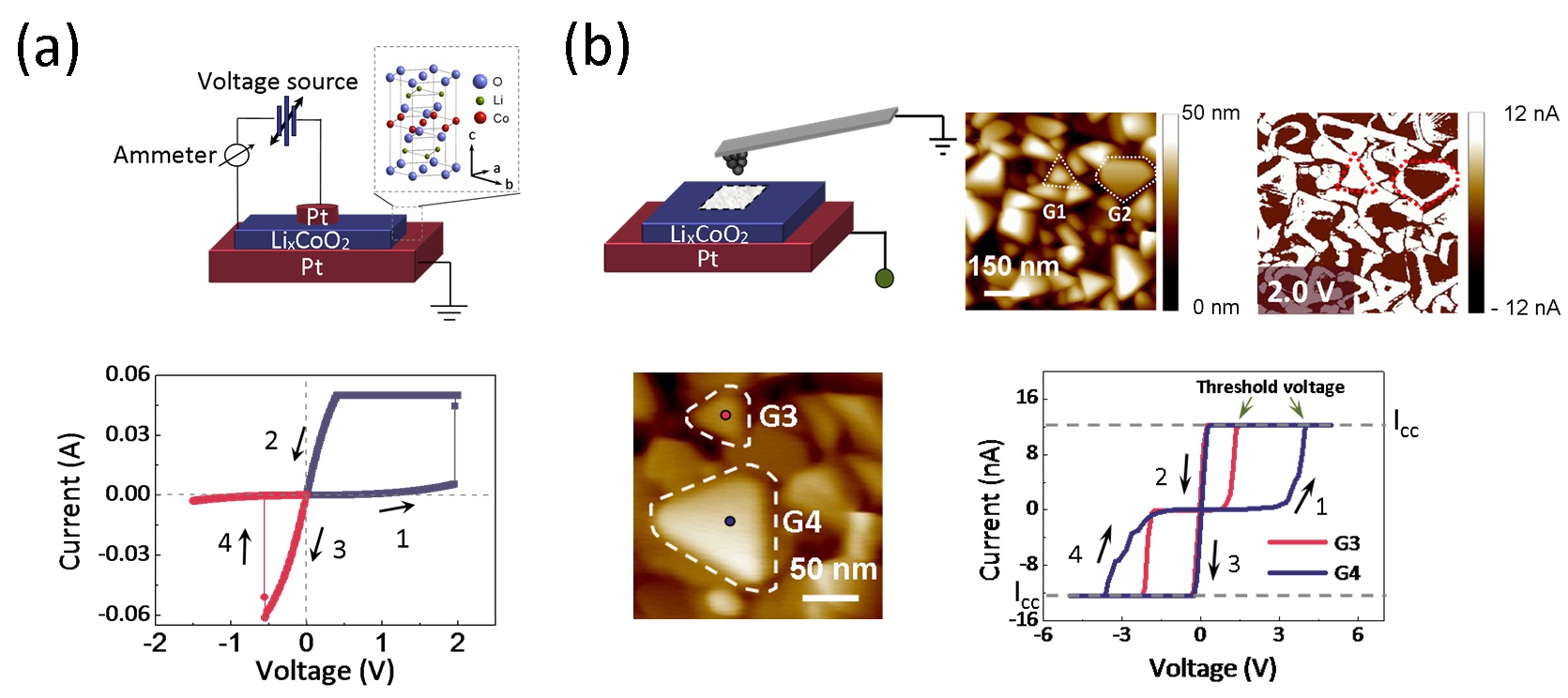
Recently, the group studied the Li-ion diffusion behaviors in LixCoO2 thin film. Firstly, they fabricated Pt/LixCoO2/Pt thin film sandwiched structures with stable resistive switching effects which confirmed closely related to Li-ion diffusion, and the resistive states of the device correspond to different contents of Li in the LixCoO2 film (Fig. 1(a)). Then the conductive atomic force microscopy (c-AFM) was employed to study the local conductance changes in the LixCoO2 films at the nanoscale (~10 nm) under an electrical field (Fig. 1(b)).
 |
|
Fig.1 (a) the resistive switching characteristics of Pt/LixCoO2/Pt device (b) the conductance change studies of LixCoO2film at the nanoscale under electrical fields by using c-AFM |
It is found that the Li-ion located near grain boundaries can easily diffuse in contrast to that in grain interiors which far away from grain boundaries, and the relationship between the Li-ion diffusion threshold voltage and grain size are further studied. Their first-principle calculation results show that the Li-ion diffusion energy barrier at grain boundaries is only 0.7 eV, much smaller than that at interiors (6.8 eV), which proved in consistent with the experimental results (Fig. 2).
 |
|
Fig. 2 (a) location dependence of Li-ion diffusion threshold voltage in LixCoO2nanograins (b) Li distribution characterization in LixCoO2nanograins after applying various bias voltages (c) current-time characteristics of grain boundary areas and interiors under various bias voltages (d) a model of Li-ion diffusion along grain boundaries for first-principle calculation |
Based on the above results, the group proposed that high-rate charge Li-ion batteries with a high capacity can be achieved by adapting LixCoO2 nanostructured electrodes composed of grains with a large grain boundary area and using a suitable charge voltage. Related results were published on Scientific Reports 3, 1084(2013)http://www.nature.com/srep/2013/130117/srep01084/full/srep01084.html .
Professor Runwei Li runweili@nimte.ac.cn
Research Group Url: http://english.nimte.cas.cn/rh/rd/mmd
All Images by ![]()

