Lithium-metal batteries offer great energy density improvement over lithium-ion, but understanding their cyclability is a daunting task. Now, an analytical method is reported to quantify lithium in its electrochemically inactive and active forms, enabling insights about the anode reversibility throughout cycling.
Increasing the energy density of today’s lithium-ion batteries (LIBs) is critical to enable electrification of weight-sensitive industries including aviation and shipping and will likewise advance technologies such as mobile electronics and electric vehicles. Among several promising candidates, lithium-metal batteries (LMBs) with metallic lithium anodes offer a prospect of achieving over 500 Wh kg-1 that nearly doubles the energy density of current LIBs.
The greatest challenge for LMBs is achieving high reversibility (>99.99%) of the lithium deposition and stripping process to rival that of graphite intercalation anodes in LIBs. To date, the understanding of lithium reversibility has been informed mostly by cycling tests paired with high-resolution microscope images. These techniques have enabled studying the effects of lithium structure, electrolyte reactivity, solid-electrolyte interphase (SEI) evolution, volume change, cell pressure, and electrolyte wetting on performance. However, it is challenging to quantify the true reversibility of lithium anodes by measurements such as coulombic efficiency (CE) because the initially present active metallic lithium can replenish lithium lost during early cycling.
Writing in Nature Energy, Zhaoping Liu, Ying Shirley Meng and colleagues from China and the United States report a multi-step analytical method to quantify the active (‘alive’) metallic lithium (Li0) remaining in the anode and distinguish it from the electrically disconnected, inactive (‘dead’) Li0. They also present a mathematical model that estimates the decline of active Li0 over time. These approaches offer a useful tool to understanding fundamental behaviours of lithium anodes, the prediction of battery lifetime, and ultimately, the development of high-performance LMBs.
Part of the quantification method’s ingenuity is that it draws on organometallic chemistry that was described nearly 70 years ago: metallic lithium reacts to completion with biphenyl in a tetrahydrofuran solvent. As shown in Fig. 1a, the active Li0 in the LMBs that are tested in the work of Liu, Meng and team are dissolved by the solvent into a colourful species. Importantly, the inactive Li0 is spared during the dissolution process due to its protective organic–insoluble SEI coating. The researchers are then able to quantify the active Li0 in solution using inductively coupled plasma optical emission spectroscopy (ICP-OES). For the remaining encapsulated inactive Li0, the researchers add water to dissolve the SEI. Water further reacts with any inactive Li0 beneath, generating H2 that is quantified by gas chromatography-mass spectrometry.
This analytical technique enables the research team to experimentally verify a mathematical model for active Li0 remaining during cycling, critical for performance. The key feature of their model is that the Li plating irreversibility (iRn where n is the cycle number) grows exponentially during cycling, that is, iRn = iR0eKn, where K is the irreversibility growth coefficient. Thus, the measured active Li0 should decrease exponentially with cycle number (Fig. 1b). Physically, K provides an essential link between the initial irreversibility (iR0) and the true reversibility needed to calculate active Li0. Assuming constant irreversibility with cycling, in contrast, leads to severe overestimation of active Li0 (Fig. 1b, dashed line).
Liu, Meng and colleagues then quantify the experimental active Li0 in many practical LMBs (fifteen 0.5 Ah multi-layered pouch cells) and explore the exponential growth in irreversibility for various cycling conditions. They find that the growth coefficient K for cells with a 0.5 C charge/discharge rate (1 C equals 600 mA) was five times that for a 0.2 C, reflecting the rate effect on performance. Determined model parameters also show the initial reversibility (100%-iR0) increasing from 99.00% to 99.52% with increasing the pressure from 100 to 800 kPa, while the corresponding irreversibility due to Li+-containing SEI product (SEI-Li+) formation decreases from 0.46% to 0.14%. From this, the authors point to the need for further understanding of SEI cracking and lithium morphology in practical LMBs.
There are many opportunities for the battery industry to build on the work of Liu, Meng and team. Their quantitative analysis may reveal phenomena in larger cell formats that aid future cell design and packaging engineering. It will be interesting to similarly quantify active Li0 evolution in long-lasting LMBs using solid state electrolytes, especially in cases where short-circuiting is no longer the dominant failure mechanism. The same approach could also be applied for studying metallic anodes with similar limited lifetimes and side reactions, such as sodium, zinc, and magnesium. The exponential expression for irreversibility growth from their work also provides an excellent starting point for LMB lifetime modelling: for instance, it may be combined with machine learning methods for more effective and efficient predictions.
It is worth mentioning that successfully separating the inactive Li0 in the approach of Liu, Meng and team relies on the stability of its encapsulating SEI, which varies significantly with electrolyte chemistry. We also note the substantial process waste from the ICP-OES sample preparation — requiring solvent evaporation followed by 180°C autoclaving with nitric acid — so there is an opportunity to minimize the harsh conditions and equipment requirements. We are also curious about industry appetite for scaling up this technique to analyse entire electrode sheets (compared with the small disk sampling used in the research work, Fig. 1a) to accelerate large format cell R&D, while recognizing the importance of scaling down to enable higher throughput benchtop analysis of smaller format cells.
In summary, Liu, Meng and team adapt multiple analysis methods, leveraging the usage of multiple phases and separation reactions, for their quantification of lithium anode reversibility. Their work highlights the importance of multidisciplinary battery engineering teams that include organometallic chemists and process engineers. These collaborations will bring novel characterization methods that are essential to the development of emerging battery technologies. (Nature Energy)
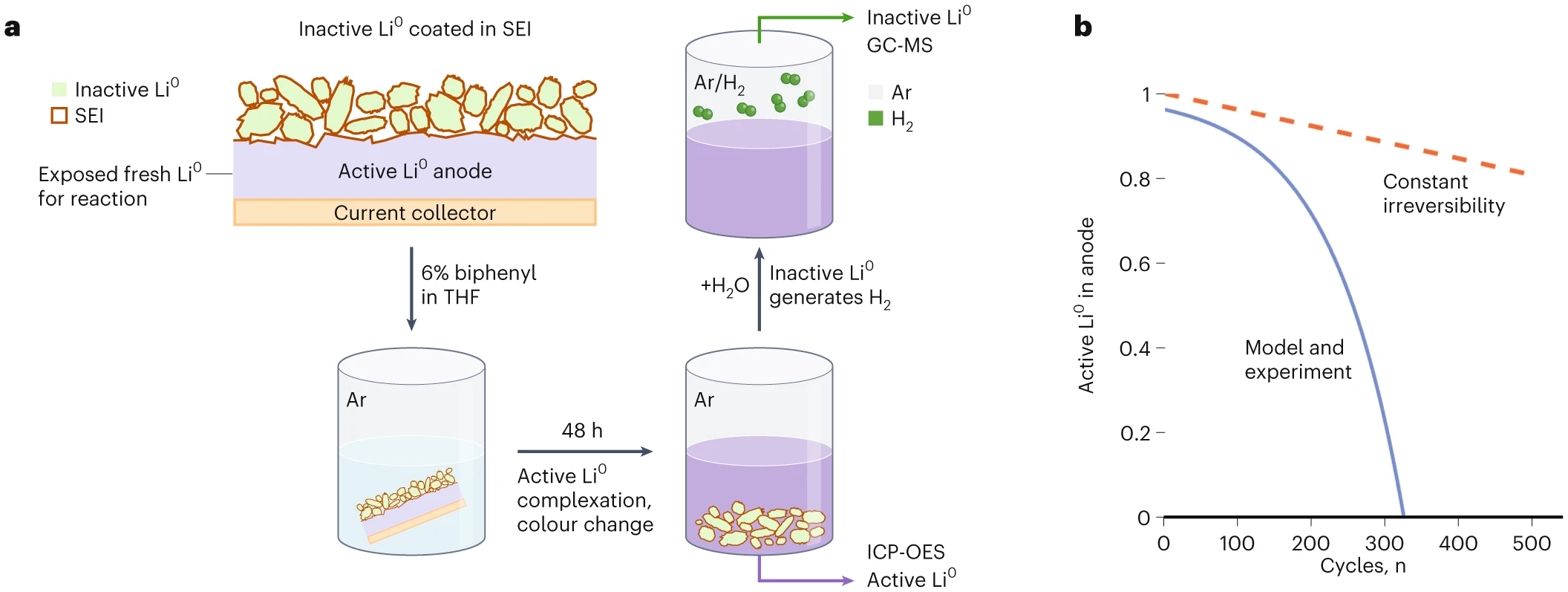
Fig. 1: Distinguishing active and inactive lithium metal (Li0) in batteries.
a, Lithium metal anode disks punched from cycled batteries contain Li0 that is connected to the battery current collector (active) and electrically isolated in small chunks due to insulating solid electrolyte interphase (inactive). Biphenyl in tetrahydrofuran (THF) solvent reacts selectively with the active Li0 via the sides where SEI-free Li0 is exposed after punching, forming the dark liquid that can be partially removed and analysed by inductively coupled plasma optical emission spectroscopy (ICP-OES). Inactive Li0 is then quantified by the hydrogen gas it forms upon exposure to water using gas chromatography-mass spectrometry (GC-MS). b, These experiments enabled validation of an exponential fitting (in blue; schematically shown) that describes how the active Li0 depletes with cycling. For comparison, assuming an unrealistic constant irreversibility leads to an overestimation of the reversibility and active Li0 remaining.

